Abstract
Abstract 2708
KSHV, also known as human herpesvirus-8, is the etiologic agent of a lymphoproliferative disorder, KSHV-associated multicentric Castleman disease (KSHV-MCD), two lymphomas: primary effusion lymphoma (PEL) and large cell lymphoma arising in KSHV-MCD (together, KSHV-NHL) and Kaposi sarcoma (KS). KSHV-associated diseases mainly occur in patients with HIV. KSHV is notable for its modulation of host immune response, including induction of IL6 and IL10; and an IL6-related KSHV-associated inflammatory cytokine syndrome (KICS) has been described in HIV/KSHV infected patients without KSHV-MCD. The clinical features and natural history of KSHV-NHL are not well understood. We hypothesize KSHV-NHL is associated with pathophysiologic features similar to those of KSHV-MCD, and is unique among HIV-associated lymphomas.
Clinical records of patients with KSHV-NHL diagnosed 2000-12, treated in the HIV and AIDS Malignancy Branch Clinic were reviewed, and evaluated for clinical, and laboratory abnormalities using our criteria for KICS (NCT01419561): at least 2 select clinical and laboratory manifestations, not otherwise explained; elevated c-reactive protein [CRP]; and KSHV viral load greater than 100 copies/106 PBMC; Additionally, serum inflammatory cytokines, (IFN-gamma, TNF-alpha, IL-1beta, IL-6, IL-8, IL-10, IL12p70; Mesoscale Discovery, Gathersburg, MD) as well as PBMC-associated KSHV viral load, platelets, hemoglobin, and albumin were evaluated in KSHV-NHL patients, and compared to patients with A) Symptomatic KSHV-MCD and no KSHV-NHL, and B) Other HIV-associated lymphomas. Comparisons used exact two-tailed Wilcoxon rank sum tests, p<0.005 was considered statistically significant, p<0.05 strong trends.
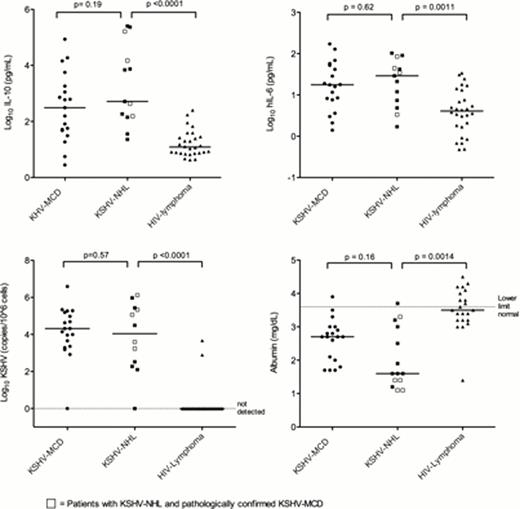
Study cohort: 14 patients with HIV and KSHV-NHL; men (13), woman (1); median (med) age 42 yrs (26, 60); African American (4), African (2), Hispanic (4), white (4). Diagnoses included PEL (12, 5 with extracavitary manifestations) and large cell lymphoma in KSHV-MCD (2). Of the 14, 5 had pathologically confirmed KSHV-MCD, and 10 had cutaneous KS. Laboratory data: med CD4 126 cells/uL (15,1072), HIV viral load <100 copies/ml (7). All 10 KSHV-NHL with complete CRP and KSHV data either met criteria for KICS or had a history of KSHV-MCD; med CRP 48.8 g/dL (4.6, 114). Controls: A) KSHV-MCD (n=19): med age 43 (29, 54), med CD4 266 (67,1319). B) Other HIV-associated lymphoma (n=28): med age 37 (21, 60); med CD4 29 cells/uL (0, 730). Laboratory, immunologic, and virologic parameters were comparable between KSHV-NHL and KSHV-MCD, with no significant differences or trends towards differences in CRP, hemoglobin, platelets, CD4 count, albumin, sodium, KSHV viral load, or any measured cytokine. Compared to patients with other HIV-associated lymphomas, those with KSHV-NHL had statistically significant elevated serum IL10 (med 513 vs 12.2 pg/ml, p<0.0001), IL6 (29 vs 4.1 pg/dl, p=0.0011), KSHV viral load (med 3913 vs. 0 copies/106 PBMC, p<0.0001), and hypoalbuminemia (med 1.8 vs 3.5 mg/dL, p=0.001) (Figure). Strong trends towards statistically significant thrombocytopenia (med 87 vs 246 K/uL, p = 0.0064), anemia (med 8.9 vs 11.1 gm/dl, p=0.019), hyponatremia (med 134 vs 137 mEq/L, p=0.03), and elevated IL1beta (1.6 vs. 0.5 pg/dL, p=0.024), and IFNgamma (med 6 vs 2.9 pg/dl, p=0.038) were also observed.
In the era of antiretroviral therapy, KSHV-NHL in HIV occurs at a broader range of CD4 counts than previously appreciated, often in the setting of suppressed HIV. Significant overlap exists between KSHV-associated lymphoproliferative disease and KSHV-NHL. Essentially all KSHV-NHL patients presented with inflammatory symptoms, elevated CRP, hypoalbuminemia, and cytopenias, and either had KSHV-MCD or met our working criteria for KICS. Elevated KSHV-infected PBMCs and associated KSHV modulation of host immune response leading to marked elevations in IL10 and IL6 likely contribute to symptoms and KSHV-NHL pathogenesis. KSHV-MCD and KICS Natural History studies are ongoing, and evaluation of curative-intent regimens for KSHV-NHL incorporating strategies to target these unique immunologic and virologic abnormalities is warranted.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal