Abstract
Abstract 2687
Patients with early stage (Stages I, II) diffuse large B cell (DLBCL) have been traditionally treated with either CHOP x6 alone or with CHOP followed by involved field external beam radiotherapy (IFRT). With the demonstration of the superiority of rituximab CHOP (RCHOP) in advanced stage DLBCL, RCHOP has been adopted as the chemoimmunotherapy standard of care in early stage disease with or without IFRT. Radioimmunotherapy (RIT) has the advantage of delivering high-energy, short path length radiation in a targeted approach using an anti-CD20 antibody as the carrier. Thus, radiation is potentially targeted to microscopic sites outside of known disease.
To evaluate the complete response (CR) rate and functional CR rate (CR or CRu/PR and PET negative) to a treatment regimen of RCHOP followed by 90Y-ibritumomab tiuxetan (Zevalin, Spectrum Pharmaceuticals) radioimmunotherapy (RIT); only patients not achieving PET negative status after RIT were to be given IFRT. The study goal was to achieve a functional CR rate of ≥75%.
Eligible patients were those with new, untreated Stages I or II DLBCL as determined by standard CT scanning, adequate blood counts, creatinine and bilirubin <2.0 mg/dl, cardiac ejection fraction >45%, and a performance status 0–2. Stage I patients were required to have ≥1 adverse risk factor (≥60 years, bulky disease, elevated LDH or PS2). Patients received standard RCHOP21 × 2 cycles and then were restaged. Patients in CR received 2 additional cycles; those in PR or CRu received 4 additional cycles. At end of RCHOP a PET scan was repeated and centrally reviewed; those with a PR or functional CR proceeded to Zevalin RIT 0.4 mCi/kg (maximum 32 mCi) within 12 weeks of the last RCHOP. Patients were restaged 12 weeks post-RIT; PET negative patients were observed without maintenance; PET positive patients received 30Gy involved field radiation and then were restaged. Descriptive statistics were used to summarize the patient characteristics, treatment and response. The Kaplan-Meier method was used to estimate failure rates.
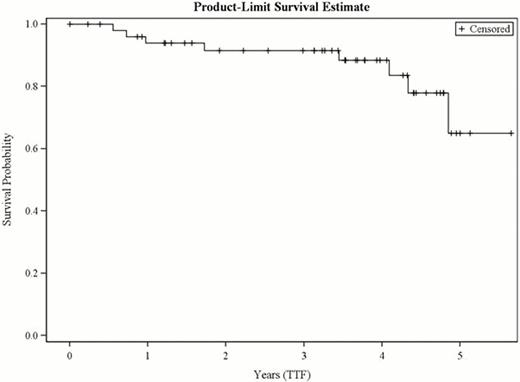
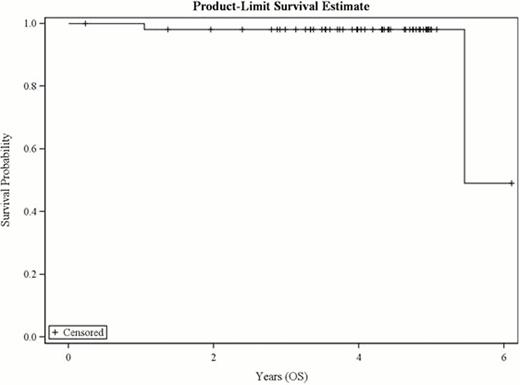
Between Dec 2004 and Nov 2008, 62 patients were enrolled; after pathology review 53 were considered eligible. 42% (22/53) patients were stage I/IE; 58% (31/53) were stage II/IIE. 57% (30/53) received 6 cycles of RCHOP; 40% (21/53) received 4 cycles; and 2 patients received 1 and 2 cycles, respectively. After immunochemotherapy, 79% (42/53) were in functional CR and 19% (10/53) PR; 1 was unevaluable. Of the 53 eligible patients who completed RCHOP therapy 91% (48/53) proceeded to RIT. After RIT, 87% (46/53; 95% CI: 75–95) were in CR/CRu and 89% (47/53; 95% CI:77–96%) were in functional CR. One patient proceeded to IFRT. The median follow-up is now 4.3 years. Seven (13%) of patients have had tumor progression but only 2 have died (1 of disease). At 4 years, 88% of patients remain progression free and 98% remain alive (Fig 1,2). The median has not been reached for either PFS or OS. There has been one case of myelodysplastic syndrome.
Combination immunochemotherapy and RIT is an active regimen for patients with early stage DLBCL. Nearly all patients will achieve functional CR without the requirement of IFRT. The regimen of RCHOP and RIT for early stage DLBCL is worthy of further study.
Witzig:Spectrum: Membership on an entity's Board of Directors or advisory committees, Research Funding, uncompensated Other. Off Label Use: Use of Zevalin in treating patients with Stage I-II DLBCL. Kahl:Roche: Consultancy; Genentech: Consultancy, Research Funding. Horning:Genentech: Employment; Roche: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal