Abstract
Abstract 2619
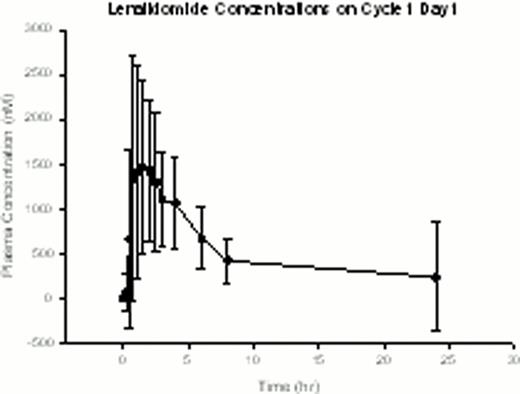
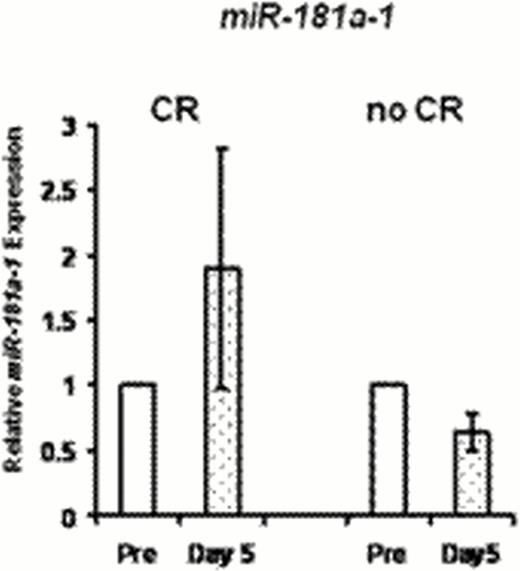
Expression of miR-181a confers better prognosis in cytogenetically normal (CN) AML (Schwind et al, JCO 2010). Laboratory studies by our group have demonstrated that miR-181a is upregulated by C/EBPα-p30. CN AML patients (pts) harboring CEBPA mutations, the majority of which block C/EBPAα-p42 expression but allow C/EBPα-p30 expression, have elevated miR-181a and more favorable clinical outcome (Marcucci et al, JCO 2008). We hypothesized that pharmacologic potentiation of C/EBPa-p30 expression, and in turn miR-181a upregulation, would increase chemosensitivity and improve clinical outcomes in AML. Based on prior in vitro and in vivo studies, LEN was selected as a candidate agent to achieve these pharmacodynamic (PD) endpoints. Pharmacokinetic (PK) and PD studies from our prior single-agent, high-dose LEN trial demonstrated upregulation of miR-181a in leukemic blasts at tolerable doses; preclinical studies showed that this effect is dose dependent. Given these results and previously demonstrated LEN clinical activity in AML (Blum et al, JCO 2010; Fehniger et al, Blood 2011; Ades et al, ASH 2010; Lancet et al, ASH 2011), a phase I trial was conducted of LEN as miR “priming” with intensive chemotherapy in two parallel cohorts of AML: Prior Therapy (PT) Cohort for relapsed/refractory pts <65 years of age, and Untreated (U) Cohort for newly diagnosed pts >60 years. In both groups, LEN was given for 14 days(d) starting at 25mg/d; cytarabine and idarubicin (starting dose 12/mg/m2 × 3d) began on d5. PT Cohort received cytarabine at a starting dose of 1.5 g/m2/d over 24 hours for 4d; U Cohort received 100mg/m2 for 7d in standard fashion. 31 pts enrolled, 17 with relapsed/refractory AML and 14 with previously untreated AML. PT Cohort pts had median age 53 years (range, 22–64) and median presenting WBC count of 4.2 × 109 (range, 0.9–22.7 × 109). U Cohort pts had median age 69 years (range, 48–77), median presenting WBC count of 3.1 × 109 (range, 0.5–31.9 × 109), and median marrow blasts of 36%. None of the U Cohort pts had favorable risk disease by ELN classification, 6 had adverse risk. Note that one patient <60 years of age was enrolled in U Cohort due to a recent amendment expanding eligibility. Dose limiting toxicities included rash (4), delayed hematologic recovery (1), mucositis (1), and cardiomyopathy (1). One pt died within 30d of starting treatment (surgical complication unrelated to treatment); however, two additional pts died of therapy-related complications within 60d of induction [intracranial hemorrhage with refractory thrombocytopenia (1) and cardiomyopathy (1)]. The maximum administered dose of LEN was 30mg/d, and we are currently expanding the 25mg dose level in both groups. PT Cohort is being expanded at LEN 25mg with idarubicin 8mg/m2/d and cytarabine at 1mg/m2/d. U Cohort is being expanded at the MTD of LEN 25mg with idarubicin 12mg/m2/d and cytarabine at 100mg/m2/d. Complete remission (CR) was achieved in 41% (7/17) in the PT Cohort and 69% (9/13, all >60 years) in the U Cohort. One pt in U Cohort was not evaluable for response as the pt discontinued treatment before chemotherapy was given. Preliminary PK parameters and LEN exposure (Figure 1) are consistent with what we reported previously. Observed PK of idarubicin was similar to expected and did not suggest an interaction with LEN. There was no apparent difference in PK of LEN in those who experienced skin rash (or other toxicities/clinical outcomes) compared with those that did not. Preliminary PD testing of LEN-induced modulation of miR-181a expression was consistent with the hypothesis of the trial. Results in a small subset of pts (N=6) tested to date showed a trend for greater post-LEN increase in miR-181a(pretreatment vs. d5 before chemotherapy began) in pts who achieved CR compared with those who did not (Figure 2). In conclusion, LEN priming with chemotherapy is reasonably well tolerated and active in AML. We are planning a randomized phase II study in previously untreated patients to test the hypotheses and compare efficacy of LEN priming vs. post-chemotherapy LEN. For the current trial, an amendment to shorten the course of LEN in order to facilitate dose escalation above 25mg is planned. Updated correlative results for all enrollees will be presented. Supported by NCI U01 CA 76576-05, NIH/NCI K23CA120708, Leukemia and Lymphoma Society SCOR 7004-11, and SPORE P50-CA140158.
Off Label Use: Lenalidomide in AML.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal