Abstract
Abstract 2611
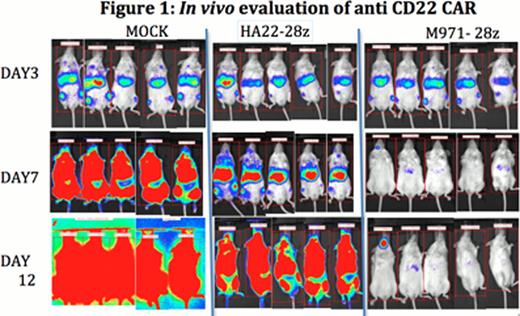
CD22 is expressed on the surface of B cell hematologic malignancies such as acute lymphoblastic leukemia (ALL). CD22 is a Siglec family lectin present on B cells, starting at the pre-B cell stage of development, but is not expressed on plasma cells. CD22 consists of 7 extracellular Ig domains and is found in 2 isoforms, one of which is missing the second and third N-terminal Ig domains. We generated CAR modified T cells containing anti-CD22 extracellular binding motifs fused to intracellular signaling domains for T cells activation (CD3 zeta) or costimulation (CD28 or 4-1BB). The binding motifs were derived from scFvs that targeted a membrane distal epitope of CD22, Ig domain 3, (BL22 and a higher affinity HA22 motif) or that bound a more membrane proximal, Ig domains 5–7, of CD22 (m971). The CAR constructs we generated were second-generation (CD28 and CD3 zeta; or, 4-1BB and CD3 zeta) or third generation (CD28, 4-1BB and CD3 zeta signaling domains). A CH2CH3 spacer domain from IgG1 was added in some constructs to examine the impact of extending the scFv-derived binding domain away from the transduced T cell membrane. In vitro cellular cytotoxicity and cytokine release experiments with 4 B cell-ALL cell lines (REH, SEM, NALM6, KOPN8) as well as the CD22 (+)ve Daudi and Raji cell lines were performed. Our results demonstrate that addition of the CH2CH3 domain did not improve tumor lysis and that standard affinity BL22 and higher affinity HA22-derived scFv epitopes were equivalent. With regard to signaling domains, second generation constructs were better than third generation constructs both in vitro and in vivo. In comparison between second generation constructs, CD28 containing domains outperformed 4-1BB with regard to lytic activity and cytokine release. Most surprising was the activity of the m971-derived scFv binding epitope. m971-CAR had significantly higher killing activity, a far more robust cytokine release profile, and superior in vivo activity. NSG mice were injected i.v. with 0.5× 106 NALM6-GL cells (pre-B cell ALL line engineered to express luciferase). Three days later, when disease was evident, mice were treated with 1×107 CAR+ T cells, and then followed by bioluminescent imaging to measure disease burden. The m971 CAR was significantly more potent at tumor clearance than our previously developed most active construct expressing the HA22-derived scFv domain (Figure 1). Disease progressed rapidly when non-transduced T cells were used (mock). We are currently examining the activity of different signaling domains on m971 CAR efficacy in vivo and directly comparing the anti-CD22 m971 CAR to the CD19 CAR currently being evaluated in clinical trials. These studies will guide future anti-CD22 CAR-based anti-leukemia immunotherapy trials.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal