Abstract
Abstract 2606
Since the FDA approval for clofarabine in the treatment of childhood relapsed or refractory acute lymphoblastic leukemia (ALL) several studies have been launched which put clofarabine under scrutiny in combination with other cytostatic drugs as second or third line therapy. As a novel treatment strategy we introduced the combination of clofarabine with asparaginase into the frontline management of ALL.
To assess safety and efficacy of clofarabine in combination with PEG-ASP, high risk B- progenitor and T-ALL patients received this regimen as a postinduction treatment within the CoALL 08–09 protocol.
In October 2010 the CoALL trial 08–09 was opened for enrollment of patients under 18 years with confirmed diagnosis of acute B-progenitor or T-cell leukemia. Until December 2011 109 patients were accrued as study patients.
Patients being identified for a high risk of relapse by PCR based MRD at the end of induction were stratified to receive the combination of clofarabine 5 × 40 mg/m2 and pegylated asparaginase (PEG-ASP) 2.500 IU/m2 at the beginning of consolidation therapy.
Criteria for eligibility were cytomorphologic remission and an MRD load ≥ 10−4for B-progenitor patients and a MRD load ≥ 10−3 at day 43, after they had already received one cyclophosphamide and methotrexate containing consolidation cycle for T-ALL patients. All other patients received the standard treatment of high dose cytarabine 4 × 3g/m2(HIDAC) in combination with PEG-ASP 2.500 IU/m2.
For comparison a historical control cohort from the predecessor CoALL 07-03 trial were analyzed who fulfilled the same MRD criteria. In this trial all patients received the standard postinduction therapy with high dose cytarabine and asparaginase.
Forty-two out of 109 patients of the CoALL 08–09 trial (39 B-progenitor and 3 T-ALL) fulfilled the inclusion criteria, were stratified and received the clofarabine/PEG-ASP treatment. No unknown or unexpected severe side effects were observed after the treatment of clofarabine with PEG-ASP. No grade 3 or 4 skin, central or peripheral neurological or renal toxicity occurred. In 13/42 patients grade 3 and in 6/42 patients grade 4 elevation of transaminases were reported, which were all reversible.
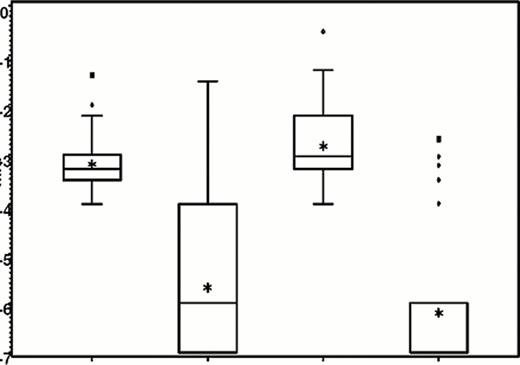
There was a remarkable response to the treatment of clofarabine/PEG-ASP measured by MRD. In comparative analysis the MRD response to clofarabine/PEG-ASP was superior to that reached after HIDAC/PEG-ASP as it is shown in Figure 1. A logistic regression analysis of the MRD response with the MRD level at end of induction as covariable showed an odds ratio of 0.26 (95% CI 0.9-0.8, p=0.02) for clofarabine vs HIDAC.
In this cohort of high risk ALL patients clofarabine in combination with PEG-ASP given as the first postinduction treatment in the frontline management of acute lymphoblastic leukemia was well tolerated without severe or persistent side effects.
Moreover, the antileukemic effect of this combination appears to be superior compared to the historical control group treated with high dose cytarabine and PEG-ASP at the same time point of treatment which warrants further investigation in a randomized fashion.
Boxplots of MRD levels prior to and after treatment with clofarabine or high dose cytarabine
Boxplots of MRD levels prior to and after treatment with clofarabine or high dose cytarabine
| HIDAC . | clofarabine . | ||
|---|---|---|---|
| before | after | before | after |
| HIDAC . | clofarabine . | ||
|---|---|---|---|
| before | after | before | after |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal