Abstract
Abstract 2204
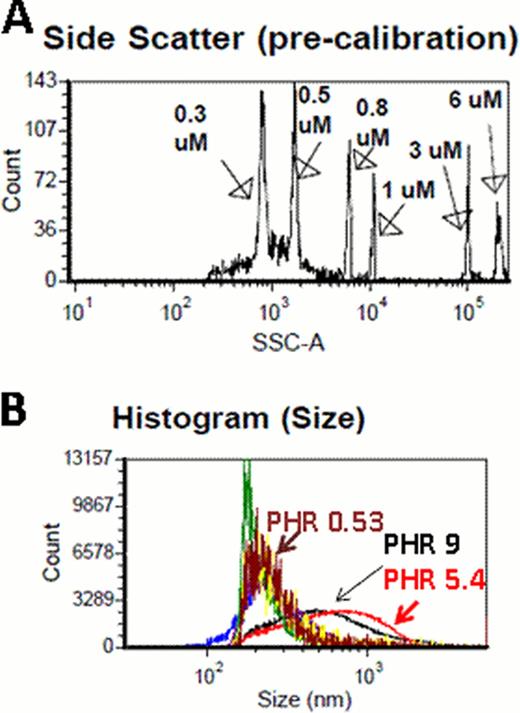
Electrostatic interactions between Platelet factor 4 (PF4), a cationic protein, and heparin, an anionic carbohydrate result in the formation of ultra-large complexes (ULCs) that are immunogenic in mice (Suvarna, Blood 2007) and contribute to the immune pathogenesis of Heparin-induced thrombocytopenia (HIT). Previous studies (Rauova, Blood 2005; Greinacher, Arterioscler Thromb Vasc Biol, 2006) have shown that the size of ULCs is determined by the concentration and the molar ratios of PF4:H (PHRs) of each compound. Size determination of PF4/H complexes has been problematic due to technical limitations of two commonly employed methods for sizing complexes, photon correlation spectroscopy (PCS) and size exclusion chromatography (SEC). PCS is a technique for measuring particles in solution using laser illumination is based on principles of Brownian motion. PCS performs optimally with monodisperse populations and is biased by the presence of large aggregates. SEC, a liquid chromatography method, is technically cumbersome, requires sample labeling and not feasible for measuring large numbers of samples. To address these limitations, we examined two novel approaches for measuring a broad range of PF4/H complex size (100–3000 nm) in vitro: Nanosight and flow cytometry (FC). Nanosight (Nanosight Ltd, Wiltshire, United Kingdom),was employed for measuring small-sized complexes using physiologic concentrations of hPF4 (10 ug/mL). Nanosight uses proprietary software to track nanoparticles (range 10–1000nm) in solution by laser illumination with real-time tracking of the motion of individual particles by a camera. Analysis parameters provided by the software include: 1) Particle size distributions displayed as histograms 2) direct visualization of particles 3) particle counting and sizing and 4) particle scatter intensity vs. count and size. For measuring intermediate to large sized particles, formed at high hPF4 concentrations (95 ug/mL), we used flow cytometry calibrated with sizing beads on side scatter channel (SSC). FC was performed using a BD LSRII cell analyzer (Becton Dickinson, Franklin Lakes, NJ), a high throughput flow analyzer with the threshold channel for SSC set to 200 and a flow rate of 1 ul per second. The instrument was calibrated using sizing beads ranging from 0.3–6 μm in size (Figure A).
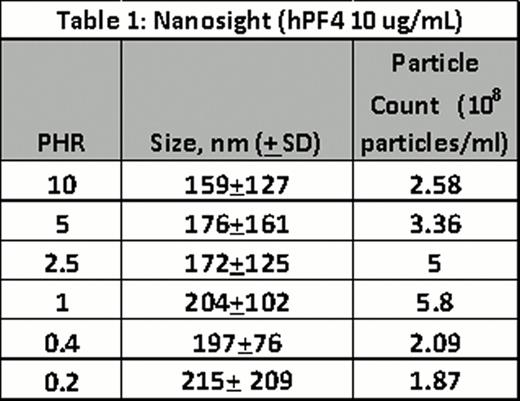
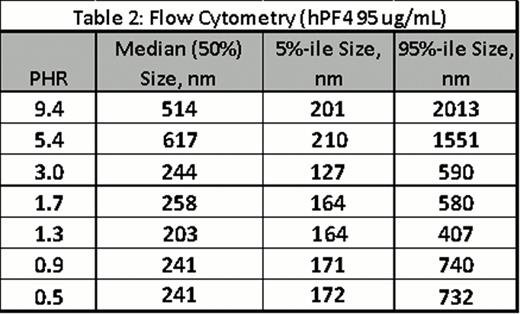
For both techniques, PF4/H ULCs were formed by adding hPF4 (10 or 95 ug/mL)and various UFH concentrations in HBSS to yield the indicated PHRs. Complexes were incubated for 60 minutes and measured by NanoSight or FC. Results of experiments using Nanosight are shown in Table 1 with results showing size and particle counts for each PHR. Results of FC are shown in Figure B and Table 2 (median, 5% and 95% size in nm). Both studies showed reproducibility for measurements for a given concentration and showed changes in complex size as a function of PHR (Figure B). Both methodologies are technically simple and provide complementary approaches to PCS for PF4/H complex size determination.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal