Abstract
Abstract 2164
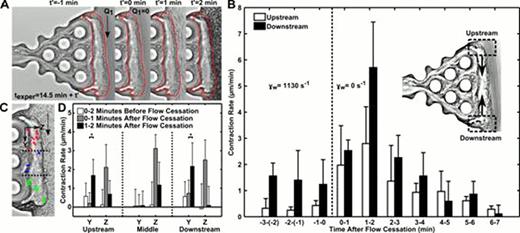
During thrombosis or hemostasis under flow conditions, platelets rapidly deposit at a site of vascular injury and quickly become mechanically connected to the vessel wall and subendothelium.1 This interwoven assembly prevents further blood loss by platelet-mediated clot contraction and stiffening.2 We utilized two microfluidic devices to investigate clot contraction dynamics in the presence or absence of transthrombus permeation (ΔP=23.5 mm Hg). Unlike many previous in vitro clot contraction studies, these devices incorporate the critical mechanical and biological thrombi properties that can only be obtained under hemodynamic conditions. In our permeation device, platelet deposits were formed for 10 min with embedded 50 nm fluorescent beads as fiduciary markers (inlet wall shear rate of 1130 s−1). Following the thrombus formation, the flow was switched without interruption to buffer for 4.5 min and then flow was completely stopped. Unexpectedly, the 20-μm thick platelet layer underwent massive platelet retraction upon flow cessation (Fig. 1A). The upstream and downstream edge contraction rates were measured throughout the buffer flow period and after flow stoppage (Fig. 1B). Contraction rates 1 to 2 min after flow cessation significantly increased by 6.5-fold (upstream region) and 4.6-fold (downstream region) (P<0.05), compared to the rate during buffer flow. The restructuring of the thrombus upstream and downstream edges by 2 min post flow cessation resulted in contractile trajectories of embedded beads towards the center of the thrombus mass (Fig. 1C). Comparisons of the time dependent contraction data in the Y and Z direction at upstream and downstream positions of the clot demonstrated increased contraction rates after flow cessation (Fig. 1D). In our rigid wall microfluidic device we investigated the role of soluble autocrine mediators. We added 1 μM SQ-29,548 (TXA2 receptor antagonist) or 10 μM MRS-2179 and 50 μM 2-MeSAMP (P2Y1 and P2Y12 platelet receptors, respectively) to examine the effects of ADP and TXA2. Following platelet deposition and 7 min of buffer/antagonist perfusion, flow was stopped and total contraction was measured with time. Both antagonists significantly reduced the total clot contraction as compared to buffer. ADP antagonists had the largest effect, reducing the total contraction nearly 75% over 7 min, whereas TXA2 antagonist reduced the contraction by 44%. These findings demonstrate ADP (from dense granules) and TXA2 from activated cyclooxygenase-1 (COX1) were the mediators of the triggered contraction response upon flow cessation. Flow dilution of these platelet autocrine mediators during intraluminal clotting balances the platelet contractile apparatus with prevailing hemodynamics, a newly defined flow sensing mechanism to regulate clot function.
Flow arrest triggers clot contraction. A thrombus formed in the absence of thrombin and presence of fluorescent 50 nm beads was rinsed with Ca2+ buffer for 4.5 min before the cessation of flow caused a rapid contraction. The outline of a pre-retracted thrombus (t'=−1 min) shows the inward retraction of the thrombus following flow stoppage (t'=0 to 2 min) (A). Contraction rate of the upstream and downstream sections of the thrombus were measured before and after flow arrest (n=3 donors) (B). Trajectories of the 50 nm beads represent the contractile response of the thrombus at upstream (red, n=6), middle (blue, n=3), and downstream (green, n=6) locations (C). Stopping the flow caused a significant increase in contraction rate in the Y and Z directions. To quantify these rates, the times before (0–2 min) and after flow cessation (0–1 min, 1–2 min) were monitored for bead velocity in the three sections of the thrombus (D). Downstream contraction rate in Y direction is shown as absolute value for contraction toward the middle region. *, P<0.01; error bars indicate mean ± SD.
Flow arrest triggers clot contraction. A thrombus formed in the absence of thrombin and presence of fluorescent 50 nm beads was rinsed with Ca2+ buffer for 4.5 min before the cessation of flow caused a rapid contraction. The outline of a pre-retracted thrombus (t'=−1 min) shows the inward retraction of the thrombus following flow stoppage (t'=0 to 2 min) (A). Contraction rate of the upstream and downstream sections of the thrombus were measured before and after flow arrest (n=3 donors) (B). Trajectories of the 50 nm beads represent the contractile response of the thrombus at upstream (red, n=6), middle (blue, n=3), and downstream (green, n=6) locations (C). Stopping the flow caused a significant increase in contraction rate in the Y and Z directions. To quantify these rates, the times before (0–2 min) and after flow cessation (0–1 min, 1–2 min) were monitored for bead velocity in the three sections of the thrombus (D). Downstream contraction rate in Y direction is shown as absolute value for contraction toward the middle region. *, P<0.01; error bars indicate mean ± SD.
No relevant conflicts of interest to declare.
References
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal