Abstract
Abstract 2163
At sites of vascular injury, activated platelets exhibit dramatic morphological changes and granule secretion to facilitate recruitment of other platelets and clot formation. In our previous work (Kita et al., 2011), we quantified the effect of the microenvironmental geometry on platelet adhesion using microcontact printing and showed that platelet adhesion and spreading is spatially regulated with microscale resolution. Here we demonstrate that platelet secretion of alpha granules, as indicated with P-selectin staining (a marker for a-granules), is also spatially regulated at the micro/nanoscale. Specifically, we show that fibrinogen micropatterns regulate and determine the spatial distribution of P-selectin at the single platelet level. We also show that tubulin, one of the major components of the platelet cytoskeleton, is also rearranged by the nanoscale geometry of the microenvironment.
Using microfabrication techniques we previously developed (Kita et al., 2011), patterned polydimethylsiloxane (PDMS) stamps were “inked” with fluorescently-labeled fibrinogen from human plasma. Fibrinogen patterns were then “microstamped” onto glass coverslips and the printed surface was blocked with 1 % BSA. 20 million/ml of washed human platelets were prepared in Tyrode's buffer and incubated onto protein micropatterned surfaces. Immunofluorescence staining was used to visualize P-selectin surface expression and intracellular distribution, and tubulin distribution of adhered platelets. Platelets were also counter-stained with a fluorescent membrane dye or phalloidin for cell detection or cytoskeleton arrangement, respectively. Images were taken via confocal microscopy with a 63x oil immersion objective.
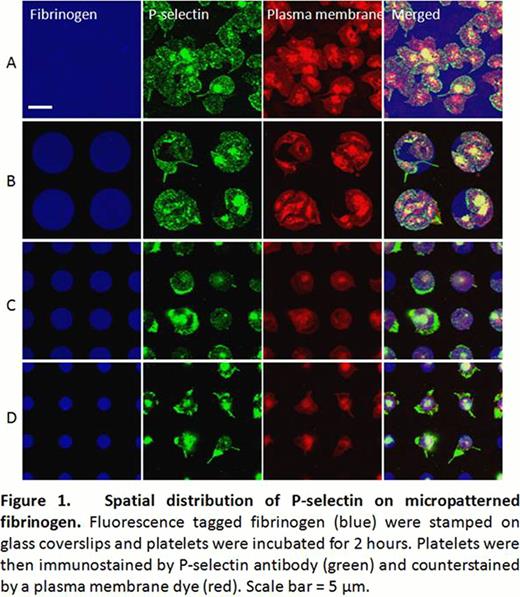
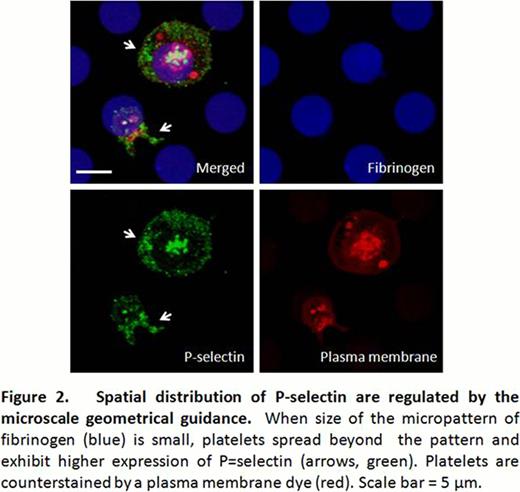
When washed platelets are incubated onto the micropatterned fibrinogen surface, they generally undergo activation and lamellipodia formation. On uniform, non-patterned fibrinogen glass surfaces, (Fig 1A), the average area of spread platelets is 24.86 μm2(n=30, standard error ± 2.27). When the fibrinogen micropattern was larger than the platelets' average area (Fig 1B), multiple platelets covered the micropattern and followed the microenvironmental geometry with high fidelity. P-selectin was detected on the entire platelet surface at slightly higher concentrations at the margins. However, when the features of the fibrinogen micropattern decreased to 5 μm in diameter (Fig 1C, D, and Fig2), platelet spreading was not constrained within the micropattern boundaries. Interestingly, the area of the platelets that spread beyond the fibrinogen micropattern exhibited much higher P-selectin expression than the areas atop the fibrinogen micropattern (Fig 2, arrows), indicating that expression and distribution of P-selectin are spatially regulated by the geometry of the fibrinogen substrate at the microscale.
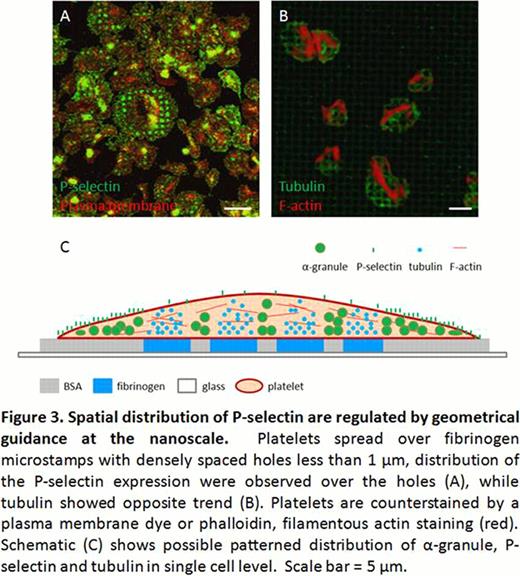
In addition, when platelets are incubated onto fibrinogen microstamps with densely spaced “holes” (0.4–1.0 μm in diameter) blocked with BSA, platelets fully spread and span over those holes. However, dense P-selectin expression was co-localized with these holes, indicating that P-selectin expression and a-granule release are spatially regulated by the underlying protein micropattern (Fig 3A). Interestingly, tubulin showed the opposite trend and only localized to areas directly above the fibrinogen micropattern (Fig 3B). These observations suggest that both platelet a-granule distribution/secretion and cytoskeleton arrangement are regulated at the single platelet level with nanoscale resolution (Fig 3C).
Using our platelet adhesion/microstamping technique, we demonstrated that platelets regulate the intracellular trafficking, distribution, and secretion of biomolecules at the nanoscale, responding to the geometry of microenvironment. We will continue to investigate how platelets spatially regulate other aspects of their physiology, including calcium signaling and distribution of receptors/ligands on different substrates such as vWF and collagen.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal