Abstract
Abstract 2024
For patients with relapsed or refractory HL, salvage chemotherapy followed by aHSCT is the standard of care. Our group previously reported excellent clinical outcomes with accelerated hyperfractionated TLI followed by high-dose chemotherapy and aHSCT (Ann of Oncol. 16:679, 2007). This strategy has been adopted as the standard at our institution for eligible individuals and we now report long-term outcomes of patients previously reported on the phase I/II clinical trial in addition to those who were subsequently treated as standard of care.
Patients with biopsy confirmed relapsed/refractory classical HL who previously received no more than 20 Gy were eligible. Salvage chemotherapy was chosen by the patient's treating physician. All patients received accelerated hyperfractionated TLI prior to transplantation administered twice daily at 150 cGy, five days/week for 10 days. The morning dose was delivered to all nodal sites including the spleen, and the afternoon dose was delivered to all sites of previous and current disease. The goal was to treat uninvolved nodal sites and spleen to 1500 cGy and sites of current and previous disease to 3000 cGy. Conditioning chemotherapy consisted of high-dose carboplatin, cyclophosphamide, and etoposide. All patients received carboplatin 450 mg/m2 by continuous intravenous infusion (CIV) on days –6 to –4 (total dose = 1350 mg/m2) and cyclophosphamide 60 mg/kg/day over 1 h on days –3 and –2 (total dose = 120 mg/kg). Patients on the phase I portion of the trial received escalating doses of etoposide by CIV from days –6 to –4. Initial dosing levels were 400 mg/m2/day, 450 mg/m2/day, 500 mg/m2/day, 600 mg/m2/day and 700 mg/m2/day. Those treated on the phase II portion of the clinical trial or subsequent to the closing of the trial were treated with etoposide 700 mg/m2/day for a total of 2100 mg/m2.
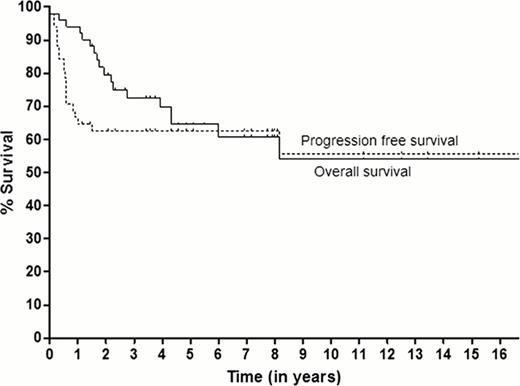
52 patients with relapsed/refractory HL at Northwestern University were treated with TLI and aHSCT from 1993 to January 2011. One patient was lost to follow-up immediately post-transplant. 51 patients were included in this analysis and had a median follow-up of 47 months (range: 0.07–204 months). Thirty patients were treated on a previously reported prospective phase I/II clinical trial. Most patients had nodular sclerosis histology (n=39, 76%) and more than half had primary induction failure (PIF; n=29). Among patients who achieved a CR with induction, 62% relapsed within one year. The most common salvage regimens were ESHAP and ICE chemotherapy and most had received two lines of chemotherapy prior to aHSCT. Only 21 patients (41%) achieved a complete response (CR) with salvage therapy and in most cases (n=31, 61%), response was determined by functional imaging prior to aHSCT. The 10-year PFS and OS for all patients were 56% and 54%, respectively. Ten-year PFS and OS for patients with PIF was 53%, compared with 63% and 59%, respectively, for those with relapsed disease (p=0.13 and p=0.20, respectively). Patients who had incomplete responses to salvage therapy had a 10-year PFS and OS of 41% and 39%, respectively, compared to 76% and 81%, respectively, for those who achieved a CR (p=0.1 and p=0.056, respectively). Treatment-related mortality within the first 100 days was observed in one patient. Five patients (10%) developed secondary malignancies; three developed MDS (one who had received MOPP induction died with MDS; one had relapsed HL post-aHSCT and died of AML and one is alive with MDS 3+ yrs post-diagnosis). There was one case each of T-cell lymphoma (7 months post-aHSCT) and melanoma.
Sequential TLI/chemotherapy conditioning for relapsed/refractory HL for patients with limited or no prior radiotherapy continues to be associated with excellent disease control and long-term survival rates including high-risk populations such as PIF and chemotherapy-resistant disease.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal