Abstract
Abstract 1889
Graft-versus-host disease (GVHD) is a frequent complication of allogeneic hematopoietic cell transplantation (HCT). While cytolytic effector function of donor-derived CD8+ cytotoxic T lymphocytes (CTLs) is critical for the elimination of the underlying malignant disease, the goal is to achieve this graft-versus-leukemia (GVL) effect without inducing GVHD. We and others showed in murine models that administration of AAT in the peri-transplant period decreased GVHD incidence and mortality. Concurrently, levels of pro-inflammatory cytokines were suppressed compared to albumin-treated recipients. Since both GVHD and GVL effects are initiated by donor cells, we asked whether donor treatment with AAT would modify GVHD or GVL effects in recipients. Of concern was the observation that AAT enhanced expression of heme oxygenase-1 (HMOX-1) which, via generation of carbon monoxide, enables the transcription factor Nrf2 to bind to the promoters of IL10 and IL1Ra, which might interfere with GVL activity.
We determined the effect of donor AAT treatment on GVHD and GVL in two murine transplant models: C3H/SW(H-2Kbc) into C57Bl/6(H-2Kb) (minor mismatch), and C57Bl/6(H-2Kb) into BALB/C (H-2Kd) (major mismatch). Four regimens of donor and recipient treatment were studied: 1) Donor albumin /recipient albumin; 2) donor albumin/recipient AAT; 3) donor AAT/recipient albumin; 4) donor AAT /recipient AAT. AAT and albumin were given to donors at 3 μg/ dose every 72 hours for two weeks before cell harvesting. Recipients received 3 μ/dose every 72 hours following total body irradiation with 800 cGy. On day 4, recipients were injected with 5 × 106 T cell-depleted bone marrow (TCD-BM) plus 5 × 105 CD4+/CD8+ splenic T cells to induce acute GVHD. To test our hypothesis that donor AAT treatment would interfere with the GVL effect, BALB/C (H-2Kd) recipients were injected i.v. with 20 × 103 A-20 lymphoma cells containing a luciferase reporter construct (A20-luc/yfp; H-2Kd), following TBI. The infusion of TCD-BM and T cells from C57Bl/6(H-2Kb) donors was delayed until day 4 to allow the tumor to home and get established.
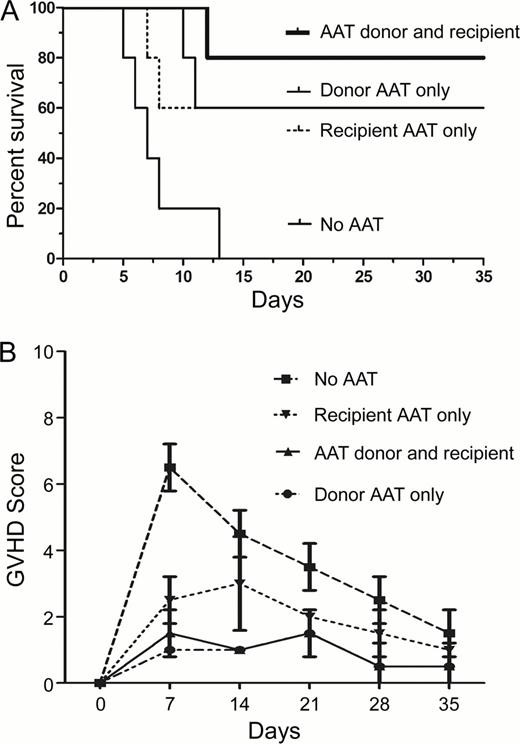
RT-PCR analysis of donor BM and T cells prior to transplantation showed up-regulation of HMOX-1 and Nrf2 (10 and 15 log2 increase, respectively), and enhanced transcription of IL-10 and IL-1Ra (50 and 10 log2, respectively, compared to albumin treated donors). In contrast, TNFa, was down-regulated (7 log2 decrease in comparison to albumin-treated donors) in both unsorted bone marrow and in CD8+/CD4+spleen cells from AAT pre-treated donors. FACS analysis revealed a 4-fold increase in frequency of CD56+NK cells, and a 5-fold increase in CD4+/CD24+FoxP3+ Treg cells, associated with a 3 fold increase in CD8+/CD205+ APCs/ DC in comparison to albumin treated donors. Mice transplanted with cells from AAT-treated donors showed lower clinical GVHD scores and decreased mortality (Figure 1) in comparison to mice transplanted from albumin-treated donors. BALB/C (H-2Kd) animals transplanted from AAT-treated donors showed significantly lower A-20 tumor signal (luciferase) intensity (over the first 3 weeks post-HCT) than did mice transplanted from albumin-treated donors (n=6, mean 4.8 × 106 (AAT) versus,7 × 106 (albumin) photons/sec/mouse, P =.03).
These data suggest that pre-transplant in vivo AAT exposure of donor cells results in protection against acute GVHD. Tumor growth was not increased in mice transplanted from AAT treated donors and, in fact, appeared to be suppressed, presumably related to a significant increase in NK cells. Further studies on donor pre-treatment or direct administration of AAT to the transplant recipients are warranted.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal