Abstract
Abstract 1816
The first-in-class proteasome inhibitor bortezomib (Bzb) has revolutionized the treatment of multiple myeloma (MM). However, Bzb often induces peripheral neuropathy (PN), which negatively affects clinical outcomes by necessitating the reduction or discontinuation Bzb treatment. If patients who are likely develop Bzb-induced PN (BiPN) could be predicted, physicians might have an additional opportunity to modify the dose regimen or reduce the Bzb dose in Bzb-based combination regimens, administer Bzb subcutaneously, or use different drugs.
We conducted a multicenter prospective study to seek biomarkers that predict BiPN. Patients with previously treated or untreated symptomatic MM received twice-weekly or weekly doses of 1.3 mg/m2 Bzb with or without dexamethasone. All patients had measurable M-protein levels. All patients provided written informed consent. Review boards at each site approved the study, which was conducted in accordance with the Declaration of Helsinki. A 2 ml sample of heparinized whole blood was obtained before treatment, as well as 2–3 days and 1–2 weeks after the first dose of i.v. administration of Bzb in the 1st cycle. Triplicate aliquots of 0.06 ml of whole blood were mixed with phytohemaglutinin (PHA), heat-aggregated IgG (HAG), lipopolysaccharide (LPS), zymosan A (ZA), or solvent phosphate buffered saline (PBS), and incubated for 4 hours at 37°C. After incubation, samples were stored at −80°C. The levels of the target mRNAs (IL2, gamma IFN, GMCSF, alpha TNF, CCL4, IL6, CXCL10, and control ACTB) were quantified from each aliquot by a previously described method (J Immunol Methods 363: 95, 2010). The analysis of mRNA and collection of clinical data were performed separately at different centers. PN was graded according to the NCI-CTCAE.
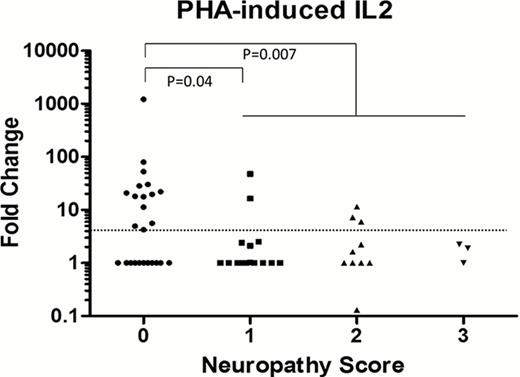
Between March 2010 and March 2012, a total of 64 patients (33 male and 31 female) were enrolled from 6 centers. The median age of all patients was 62 years. The original protocol stipulated that only relapsed or refractory patients were eligible, but in October 2011, the protocol was amended to include newly diagnosed patients due to changes in the Japanese National Health Insurance policy. Consequently, 42 patients were previously treated and 22 patients were untreated. After excluding 1 patient each for loss of consciousness or early death due to progressive disease, 62 patients were eligible for toxicity analysis. Of the 59 patients without PN before enrollment, 18 (1 of 18), 9 (8 of 9), and 4 (3 of 4) developed Grade (G)1, G2, and G3 BiPN (with pain), respectively. After treatment with Bzb, the symptoms of 2 patients who had G1 PN prior to treatment worsened to G2 PN. No change was observed in 1 patient with G2 PN. The median follow-up time of estimable patients was 165.5 (range, 31 to 666) days. A total of 2,444 (64 [patients] × 5 [stimulants] × 3 [triplicate] × 3 [time points] with some sampling failure subtracted) mRNA/cDNA samples were prepared, and 19,552 (2,444 [cDNA] × 8 [mRNAs]) PCRs were performed. In total, 53 patient blood samples qualified for mRNA analysis. In the 32 sample dataset (4 stimulants × 8 mRNAs) obtained before Bzb treatment, the BiPN grade correlated with the PHA-induced IL2, gamma IFN, and alpha TNF, and LPS-induced IL6 levels. More importantly, out of 29 patients with a more than 3-fold increase in PHA-induced IL2, 14 patients were BiPN non-inducers (73.7% prediction of non-inducer), whereas out of 34 patients with a less than 3-fold increase in PHA-induced IL2, 23 were BiPN inducers (67.6% prediction of inducers). The mRNA data from blood samples obtained after Bzb treatment did not show any substantial correlation to BiPN.
A PHA-induced increase in IL2 in peripheral whole blood obtained before Bzb administration is a promising biomarker for predicting patients who are BiPN inducers and non-inducers.
Mitsuhashi:the company who owns the technology of mRNA analysis used in this study: Employment. Abe:Janssen Pharmaceutical K.K.: Honoraria, Research Funding. Nakagawa:Janssen Pharmaceutical K.K.: Honoraria. Nakamura:Janssen Pharmaceutical K.K.: Honoraria. Iida:Janssen Pharmaceutical K.K.: Honoraria. Kizaki:Janssen Pharmaceutical K.K.: Honoraria.
This work was supported by the National Cancer Center Research and Development Fund, Japan (21-8-5) for T.W.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal