Abstract
Abstract 1731
Symptom burden in primary, post-ET and post-PV myelofibrosis (MF) is frequently severe and correlates with a poor prognosis. However, symptom manifestations are heterogeneous with variable presence of specific symptoms, splenomegaly and cytopenias. We sought to identify the spectrum and features of MF symptomatic phenotypes by cluster analysis of prospectively gathered information on MF symptoms and disease features.
Data was collected among an international cohort of subjects with MF. Data included demographics, disease features and completion of the Brief Fatigue Inventory (BFI) and Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score (MPN-SAF TSS) (Blood 2011; 118:401–408). Surveyed symptoms addressed key disease features on a 0 (absent) to 10 (worst-imaginable) scale. Cluster development was based on consideration of r-squared in hierarchical clustering using Ward's linkage. Final cluster assignment was based on the nonhierarchical k-means method. Comparisons between symptom clusters were based on ANOVA and chi-squared tests.
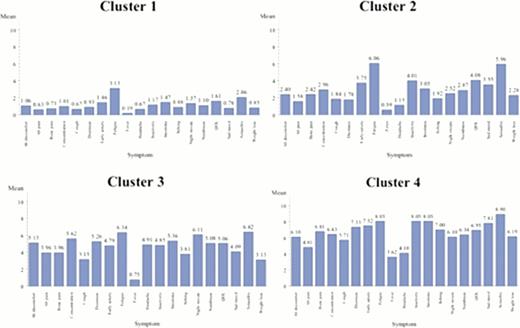
Data from 329 prospectively enrolled persons with MF was collected (Chinese 102, French 54, German 19, Italian 22, Dutch 45, English 51, Spanish 29, Swedish 7) including 223 PMF, 67 post-ET MF and 39 post-PV MF patients. Participants were of typical age (mean 59) and gender (47% F). Among all participants, four natural symptom clusters were identified (Figure 1). Among clusters, disease features including leukopenia, thrombocytopenia, and enlarged spleen varied significantly between clusters (P<0.05).
The “Fatigue Dominant with Few Lab Abnormalities” Profile (n=150 (46%; 69% PMF, 20% post-ET MF, 11% post-PV MF)). Cluster 1, the largest, is characterized by fatigue-dominant complaints in the setting of the lowest overall MPN-SAF TSS and highest proportion of males (59%). Individuals among this group have the lowest prevalence of laboratory abnormalities (65% total; anemia, 67%; thrombocytopenia, 20%) or clinical deficiencies including enlarged spleen (average 6.0 cm below costal margin), prior thrombosis (9%), prior hemorrhage (5%) or prior RBC-transfusions (20.4%). Interestingly, individuals in this group are most likely to have had prior splenectomy (5.8%).
The “Cognitive Complaints with Enlarged Spleen” Cluster (n=105 (32%; 65% PMF, 20% post-ET MF, 15% post-PV MF)). Cluster 2 is the 2nd largest cluster. Subjects have relatively few abnormal lab values (67% vs 65%–77%) but have high severity of fatigue, sexual difficulties, insomnia, inactivity and reduced QOL. These individuals have the largest spleen size (8.7cm below costal margin).
The “Nighttime and Cognitive Complaints” Group (n=53 (16%; 64% PMF, 25% post-ET MF, 11% post-PV MF)). Cluster 3 is the smallest cluster. Subjects have many cognitive and nighttime-related complaints including sexual difficulties, night sweats, insomnia, and concentration problems. Subjects with post-ET MF are predominant. This cluster also has the 2ndsmallest spleen size (7 cm) or history of prior thrombosis (9.6%), hemorrhage (7.8%) or requirement for transfusions (21.2%).
The “Severe Fatigue with Few End-organ Complaints” Cluster (n=21 (6%; 81% PMF, 14% post-ET MF, 5% post-PV MF)). Cluster 4 is the most symptomatic cohort with the highest proportion of subjects with PM. There is a lower frequency of end-organ complaints including abdominal pain, cough, and headaches. Symptoms including sexual difficulties, sad mood and insomnia are predominant. No subjects had prior splenectomy. Subjects also have the highest prevalence of prior thrombosis (29%), hemorrhage (14%), and transfusions (43%). Additionally, this cohort has the largest prevalence of lab abnormalities (77%) with thrombocytopenia (71%), leukopenia (41%) and anemia (41%).
This analysis will allow us to examine a new framework for evaluating persons with MF using symptom profiles and is the 1st cluster evaluation of MF. Lab and physical findings contrast significantly between symptom clusters indicating these phenotypic symptoms likely result from etiological factors present in specific disease phenotypes. Future studies should evaluate whether there is a correlation between cluster profiles, prognosis and genotype.
Kiladjian:Celgene: Research Funding; Novartis: Honoraria, Research Funding; Shire: Honoraria. Roy:Novartis, BMS: Speakers Bureau. Harrison:Novartis: Honoraria, Research Funding, Speakers Bureau; YM Bioscience: Consultancy, Honoraria; Sanofi Aventis: Honoraria; Shire: Honoraria, Research Funding. Vannucchi:Novartis: Membership on an entity's Board of Directors or advisory committees. Passamonti:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees. Mesa:Incyte: Research Funding; Lilly: Research Funding; Sanofi: Research Funding; NS Pharma: Research Funding; YM Bioscience: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal