Abstract
Abstract 1726
We previously reported that symptom burden among persons with ET and PV can be severe and adversely affect QOL. The presence of severe symptoms is linked to poor prognosis. There is considerable inter-subject heterogeneity regarding which symptoms are present in which subjects. No studies have empirically evaluated whether disease characteristics can be grouped in related symptom clusters. Using our previously validated 18 item Myeloproliferative Neoplasm Assessment Form (MPN-SAF) (Blood 2011;118:401–408) given in conjunction with the 9 item Brief Fatigue Inventory (BFI) (Cancer 1999;85:1186–1196), we sought to evaluate symptom burden by means of cluster analysis.
Data was collected from an international cohort of subjects with MPNs including demographics, disease features and the completed BFI and MPN-SAF instruments. Surveyed symptoms included fatigue, early satiety, abdominal pain and discomfort, inactivity, headaches, concentration, dizziness, extremity tingling, insomnia, sexual difficulties, mood changes, cough, night sweats, pruritus, bone pain and fever on a 0 (absent) to 10 (worst-imaginable) scale. Development of symptom clusters was based on consideration of r-squared in hierarchical clustering using Ward linkage. Final cluster assignment was based on the nonhierarchical k-means method. Comparisons between symptom clusters were based on ANOVA and chi-squared tests.
Data from 1,141 subjects with PV (N=519) and ET (N=622) was prospectively collected (Chinese 236, French 305, German 45, Italian 114, Dutch 191, English 56, Spanish 109, Swedish 85. Age (mean 59, range, 26–87) and gender (54% F) were typical. Five clusters were selected (Figure 1). Frequencies of prior bleeding, spleen size, anemia, presence of any lab abnormality, language, gender, and MPN type varied significantly between clusters (P<0.05).
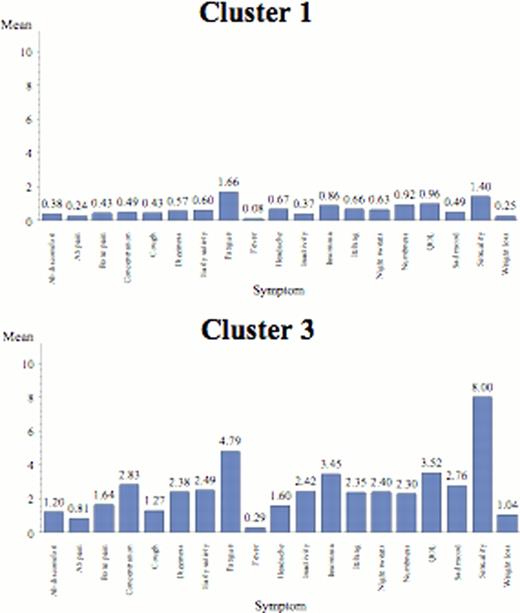
The “Reduced Symptom” Profile (n=421 (37%; 60% ET, 40% PV) The largest cluster, subjects had increased complaints of sexual difficulties and fatigue. There was a slightly higher proportion of subjects with ET (60%) versus PV. There were fewer lab abnormalities (28% prevalence) and less prior bleeding (3%) compared to other clusters. Spleen size was smallest of the cluster (1 cm below costal margin).
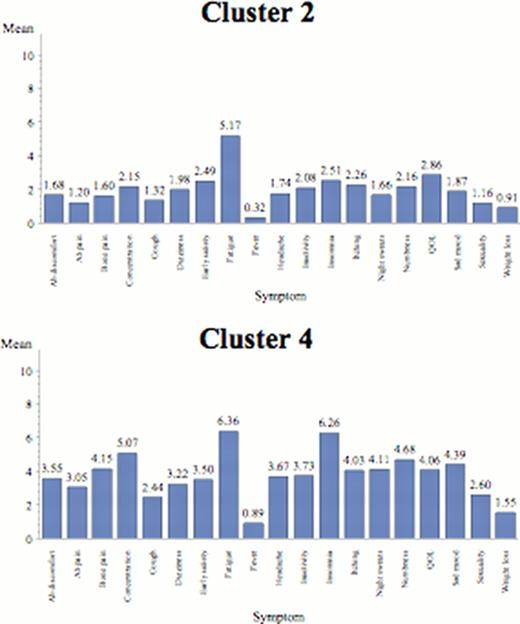
The “Fatigue-dominant” Group (n=286 (25%; 56% ET, 44% PV)). Subjects in this cluster were predominantly female and had relatively few laboratory abnormalities (19%) than other cohorts. They are characterized by high severity of fatigue compared to end-organ symptoms. Symptom profiles emphasize fatigue, QOL and insomnia with some end-organ complaints. The cohor 63% of the cohort.
The “End-Organ Complaints” Group (n=210 (18%; 49% ET, 51% PV)). Male predominant (56%), subjects had mainly macro-vascular symptom complaints including sexual difficulties, insomnia, and overall QOL, with few microvascular related symptoms (low itching/night sweats).
“Cognitive Complaints” Cluster (n=110 (10%; 53% ET, 47% PV)). The smallest cluster and female predominant (64%), main complaints include fatigue, insomnia, loss of concentration, numbness, and sad mood.
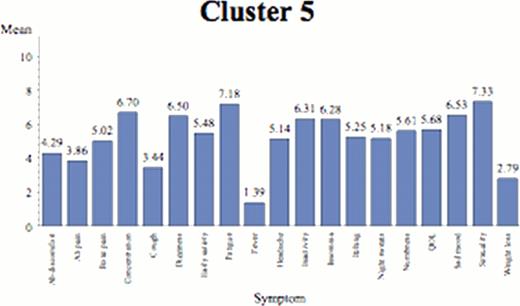
The “Highly Symptomatic” Cluster (n=114 (10%; 44% ET, 56% PV)). Subjects had many cognitive complaints and symptoms correlated with severe micro-vascular abnormalities (pruritus) and or splenomegaly. This cluster had the largest spleen sizes (mean 3 cm), the highest prevalence of prior thrombosis (29%), and highest frequency of lab abnormalities (43%). Cognitive and end-organ complaints were rated as most severe.
This analysis offers new means of evaluating persons with PV and ET utilizing symptom clusters. Laboratory and physical abnormalities differed significantly between symptom clusters indicating that our groupings likely result from biological alterations present in specific disease phenotypes. Future studies should investigate correlations between clusters and prognosis and genotype.
Individual symptom means by cluster.
Kiladjian:Celgene: Research Funding; Novartis: Honoraria, Research Funding; Shire: Honoraria. Roy:Novartis, BMS: Speakers Bureau. Harrison:Novartis: Honoraria, Research Funding, Speakers Bureau; YM Bioscience: Consultancy, Honoraria; Sanofi Aventis: Honoraria; Shire: Honoraria, Research Funding. Vannucchi:Novartis: Membership on an entity's Board of Directors or advisory committees. Passamonti:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees. Mesa:Incyte: Research Funding; Lilly: Research Funding; Sanofi: Research Funding; NS Pharma: Research Funding; YM Bioscience: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal