Abstract
Abstract 169
Approximately half of all patients with sporadic MDS will present with one of the known recurrent genetic alterations including additions/deletions of large chromosomal segments or mutations in single genes. Familial cases of MDS are rare, however, and the mutations that have been identified are not unique to MDS. Here we describe a family in which three of nine siblings were affected with early-onset MDS and a del(20) karyotype (Table 1). One of the brothers progressed to AML and died while the remaining 2 brothers are stable.
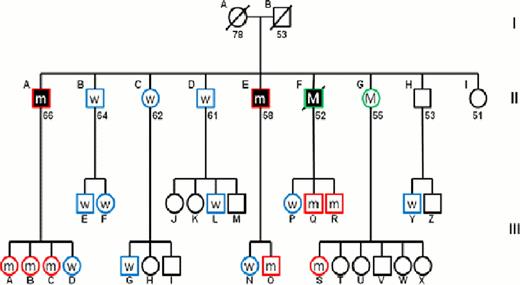
We performed whole exome sequencing on 2 affected brothers and 2 normal male family members followed by Sanger sequencing. Comparison of non-synonymous mutations common to the 2 brothers with MDS but not found in the normal controls revealed a mutated gene, Dido1, which is located at 20q13.33 upstream of the deleted region in the MDS brothers. The G to A mutation leads to substitution of a bulky basic, polar arginine in place of a small neutral non-polar glycine at amino acid 956 of the protein. This mutation, confirmed by PCR, was present in one allele in both the marrow and T-cells (not part of the MDS clone) of both brothers with MDS (Figure 1) showing that the mutation is not somatic but is, rather, a germline mutation. Since all three affected brothers harbored a del(20), it was of interest to investigate whether other patients with MDS or MDS/MPD and del(20)(q11.2) may also have this specific mutation. DNA from bone marrow mononuclear cells (BMMNC) of 10 del(20q) patients with either MDS or MDS/MPD was examined by PCR and Sanger sequencing. No mutation was found in any of the samples, suggesting that the mutation is unique to the affected family. BMMNC were available for 16 additional members of this extensive family. PCR followed by sequencing showed that 7/16 members of generation III have the identical heterozygous mutation. Interestingly, while no DNA was available from the brother who died of AML, analysis of his children revealed that 2 sons (III- Q and III-R) carry the mutation, confirming that the father also carried this mutation. Similarly, while DNA from sister II-G was not available for analysis, one of her daughters carries the mutation confirming the presence of mutation in the mother.
Death inducer-obliterator 1 (Dido1) is a putative transcription factor originally identified in a screen for apoptosis related genes in a murine pre-B cell line. The gene encodes three alternate isoforms and gene targeting of all three isoforms in a murine model leads to development of a transplantable myeloid disorder with features similar to MDS/MPN. These studies also showed variable decreased Dido1 expression in a subset of patients with myeloid disorders when compared to healthy controls. Our results support the hypothesis that Dido1 aberrations in hematopoietic stem cells may contribute to MDS evolution. The fact that deletions in the long arm of chromosome 20 are one of the most frequent aberrations found in MDS and clearly was a secondary event in the 3 affected brothers of this family suggests that Dido1 may ontribute to this specific genomic instability. Generation III are still young, but those with the mutation may have an increased susceptibility to myeloid disease and should be clinically followed.
The identification of the Dido1 mutation in this family suggests a novel pathogenic mechanism for the development of familial MDS.
Clinical features of brothers with MDS
| Brothers . | Age . | FAB . | IPSS . | Karyotype . |
|---|---|---|---|---|
| II-A | 66 | RA | Int-1 | 46,XY,del(20)(q11.2q13.3)[17]/47,idem,+8[3]] |
| II-E | 58 | RA | Low | 46,XY,del(20)(q11.2)[6]/46,XY[14] |
| II-F | 52 | RAEB-2 | High | 6,XY,-4,-5,-7,-9,-10,-12,-16,-17,-18,-20[11]/ 36,XY,idem,add(2)(q33),add(11)(p14)[6]?46,XY[cp3] |
| Brothers . | Age . | FAB . | IPSS . | Karyotype . |
|---|---|---|---|---|
| II-A | 66 | RA | Int-1 | 46,XY,del(20)(q11.2q13.3)[17]/47,idem,+8[3]] |
| II-E | 58 | RA | Low | 46,XY,del(20)(q11.2)[6]/46,XY[14] |
| II-F | 52 | RAEB-2 | High | 6,XY,-4,-5,-7,-9,-10,-12,-16,-17,-18,-20[11]/ 36,XY,idem,add(2)(q33),add(11)(p14)[6]?46,XY[cp3] |
Pedigree of the MDS/AML family. Circles represent women and squares represent men. Filled shapes indicate individuals affected with MDS/AML. Slashes indicate deceased individuals and numbers below shapes indicate present age or age at death. Roman numerals to the right of the pedigree indicate generation and letters indicate individuals within a generation. W indicates individuals who are wild-type for Dido,m indicates individuals with a heterozygous Dido mutation and M indicates individuals with inferred heterozygous Dido mutation. Empty shapes indicates individuals not sequenced.
Pedigree of the MDS/AML family. Circles represent women and squares represent men. Filled shapes indicate individuals affected with MDS/AML. Slashes indicate deceased individuals and numbers below shapes indicate present age or age at death. Roman numerals to the right of the pedigree indicate generation and letters indicate individuals within a generation. W indicates individuals who are wild-type for Dido,m indicates individuals with a heterozygous Dido mutation and M indicates individuals with inferred heterozygous Dido mutation. Empty shapes indicates individuals not sequenced.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal