Abstract
Abstract 1681
Recently the European LeukemiaNet has developed a new scoring system (European Treatment and Outcome Study [EUTOS] score) for newly diagnosed chronic myeloid leukemia (CML) in chronic phase (CP) treated with imatinib. The EUTOS score classifies patients in high or low risk on the basis of the percentage of basophils in the peripheral blood and the spleen size at diagnosis, with significant correlations with the achievement of an 18-month complete cytogenetic response (CCyR) and progression-free survival (PFS). The aim of this work was to evaluate EUTOS score in CML-CP treated in our center with imatinib as a predictive factor for overall survival (OS), event-free survival (EFS) and PFS.
Between February 2003 and May 2012 consecutive patients with newly diagnosed CML-CP were treated with imatinib 400 mg daily (n= 144) or imatinib 600–800 mg daily (n= 14) were included in the analysis. The criteria recommended by European LeukemiaNet (ELN) were used for the definitions of CCyR and the progression to accelerated phase (AP) or blast phase (BP). The EUTOS score was defined by (7×basophils) plus (4×spleen size) at diagnostic. A EUTOS score of more than 87 indicates high risk, and less than or equal to 87 low risk. EFS was measured from the start of imatinib treatment to the date of any of the following events: death from any cause at any time, loss of complete hematologic response, loss of CCyR, or progression to AP or BP. PFS was measured from the start of treatment to the date progression to AP or BP, last follow-up, or death from any cause. Survival probabilities were estimated by the Kaplan-Meier method and compared by the log-rank test whereas it was applied cumulative incidence for the probability to achieve CCyR.
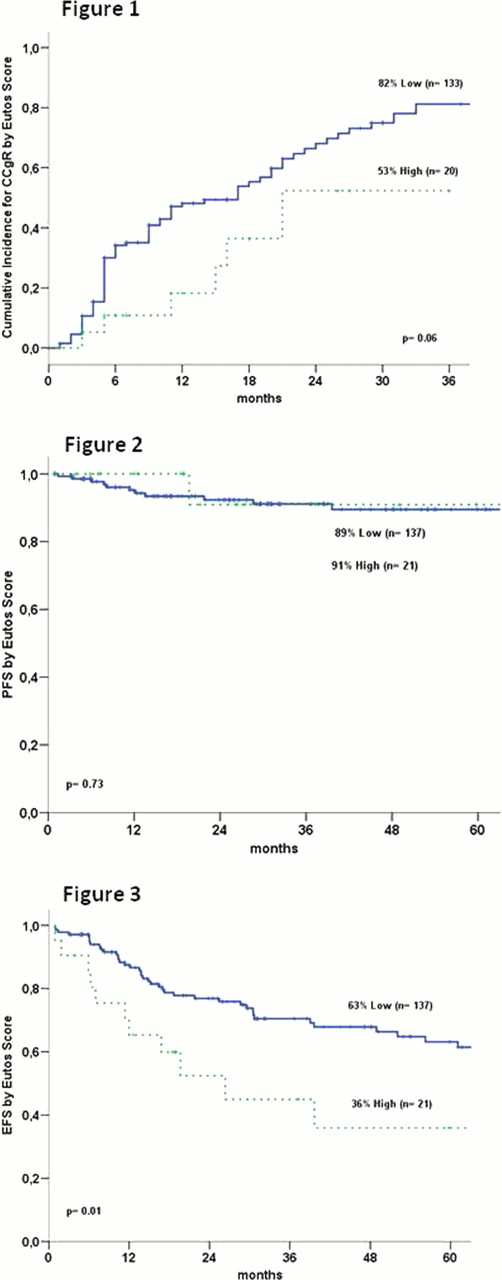
A total of 158 patients were treated, 94 (59.5%) male. The median age at Imatinib was 47 years (17–86 years). The median time from diagnosis to TKI therapy was 2 (0–6) months, with 153 (96.8%) receiving previous treatment with Hydrea. The median follow-up was 29 (1–110) months. The median splenomegaly size was 4 (0–29) cm and the median basophil percentage was 2.5% (0–18%). According to the Sokal score, 43 (34,4%) patients, 46 (36,8%), and 36 (28,8%) were in low, intermediate, and high Sokal score category, respectively (33 not available). Concerning the Hasford score, 60 (48,3%), 50 (40,4%) and 14 (11,3%) were in low, intermediate and high risk categories (34 NA). A total of 137 (86,8%) patients were in the low EUTOS score category and 21 (13,2%) in the high risk category. The cumulative probability of achieving a CCyR and MMR at 36 months for all patients was 78% and 64%, respectively. Patients who had not achieved CCyR after 6 months (51/153 – 33%) had a 2% risk of subsequent progression, which increased to 12% after 12 months, 14% in 18 months and 19% after 24 months. EFS, PFS, and OS rates for the whole group were 60%, 89%, and 92%, respectively. Patients with a low EUTOS score had higher rates of cumulative CCyR compared with patients with high EUTOS score (82% vs. 53%, p= 0.06) (figure 1). There were no differences in the cumulative CCyR rates in patients stratified by Sokal or Hasford scores (and 0.21 and P=0.82, respectively). Patients with CCyR at 18 months had a higher EFS (81% vs. 18%, p< 0.0001) and PFS rates (96% vs. 82%, p= 0.03). There was no difference in PFS (figure 2) and OS rates between patients with low and high EUTOS score. However, patients with high and intermediate Sokal score had an inferior PFS rates in comparison with low risk group (77%, 84% and 100%, respectively, p= 0.02). There was a superior EFS rates in low risk in comparison with high EUTOS score (63% vs. 36%, p= 0.01) (figure 3), whereas the overall survival there was no difference (91% vs. 100%). Sokal scores EFS rates were 68%, 60% and 40% for low, intermediate and high risk groups respectively (p= 0.03).
In conclusion, similarly to the original report, EUTOS score seems to predict CCyR, but not PFS in our population. However, EFS was significantly better in the low than high risk group. The score can discriminate patients with poor outcome, with lower probability of achieving responses to first line imatinib therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal