Abstract
Abstract 1627
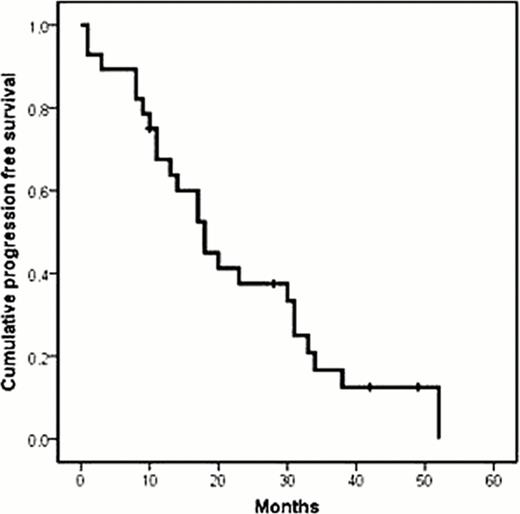
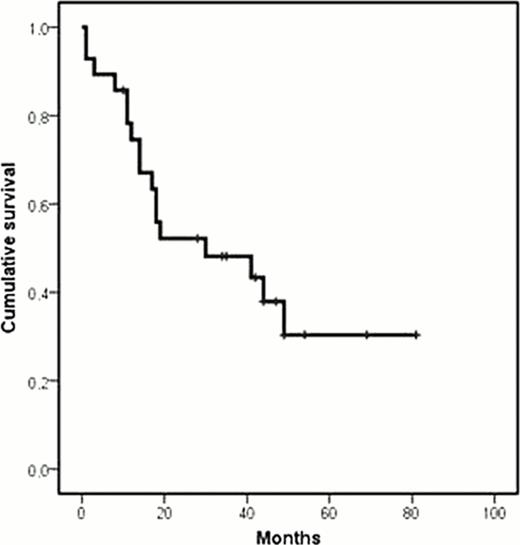
Front line treatment with chemoimmunotherapy in mantle cell lymphoma (MCL) is able to obtain a high CR rate. Regrettably, all patients with MCL eventually relapse, a situation for which no effective therapies are available. Gemcitabine plus Oxaliplatin have shown promising results in “in vitro” and in clinical studies for several types of lymphoma, its activity being improved by the addition of rituximab. Against this background, we conducted a pilot study aimed to assess the efficacy and toxicity of the combination of Rituximab, Gemcitabine and Oxaliplatin (R-GemOx) in pts with relapsed/refractory MCL. Inclusion criteria were age > 18 years and a histologically proved MCL that relapsed or was refractory to previous treatment. Patients were excluded if they had neutrophil count < 1,500 μL, platelet count < 100,000 μL, creatinine > 2.5 mg/dL, > 3 times the upper limit of laboratory normal for AST and ALT, or bilirubin > 3 mg/dL. The regimen consisted of Rituximab 375 mg/m2 day 1, Gemcitabine 1000 mg/ m2 and Oxaliplatin 100 mg/ m2 day 2, every 14 days for a total of 8 cycles. Dose and interval adjustment was done according hematological and extrahematological toxicities. Twenty-eight patients (71% male, median age 68 years, range 41–84 years) were included in this study. Median number of previous treatments was one (range: 1–4). Fifteen (53%) pts had relapsed after previous CR/uCR, 10 (36%) had progressed after a PR, and three (11%) were refractory to the previous treatment. Bone marrow infiltration was observed in 85% of evaluated patients. Median number of cycles administered was 8 (range 2–8). Toxicity was mainly hematological, with a grade 3–4 neutropenia and thrombocytopenia observed in 9 (4.7%) and five (2.6%) cycles, respectively. Main non-hematologic toxicities were hepatotoxicity grade 1–2(21%), sensitive neurotoxicity grade 1–2 (43%), and nefrotoxicity grade 2 (4%). Dose reduction was performed in only two pts for a total of eight cycles, and treatment was delayed in six pts for a total of 17 cycles. After completion of treatment, 21 pts (75%) achieved a CR/uCR, 1 (3.5%) a PR, 1 (3.6%) SD and 5 (17.8%) progressed. Stem-cell transplantation was subsequently performed in nine pts (6 allogeneic and 3 autologous). After a median follow-up of 23 months, 4 patients are alive without progression, 7 relapsed, and 17 died (13 due to progression, 2 due to aGVHD, and 2 due to post-transplant infection). Median PFS and OS of this series were 18 and 30 months, respectively. In conclusion, the R-GemOx combination showed an encouraging efficacy in relapsed/refractory pts with MCL. Hematological and non-hematological toxicity were mild. This is a feasible combination to be employed in salvaging pts prior stem-cell transplantation. Based on the above mentioned data from this pilot study, a national prospective multicenter phase II clinical trial is currently ongoing.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal