Abstract
Oral azacitidine (CC-486) is bioavailable, biologically and clinically active, and well tolerated in patients (pts) with myelodysplastic syndromes (MDS) and acute myeloid leukemia (Garcia-Manero, J Clin Oncol, 2011). Because DNA methyltransferase (DNMT) inhibitors require active cell cycling to effect methylation reversal, prolonged administration of lower doses of azacitidine may provide more extensive reversal of DNA methylation compared with shorter administration.
We conducted analyses 1) to determine the pharmacokinetic (PK) and pharmacodynamic (PD; ie, DNA demethylating activity) profiles following subcutaneous (SC) AZA and various dosing schedules of oral azacitidine; 2) to assess the correlation between the PK and PD profiles of oral azacitidine administered in extended dosing schedules; and 3) to compare PD effects observed at different time points in the 28-day (d) treatment cycle with SC AZA or oral azacitidine administered for 7 days vs. the PD effects of 300mg QD oral azacitidine administered in extended dosing schedules in pts with MDS.
This multicenter, phase 1 study had 2 parts. In Part 1, 41 pts with MDS, CMML, or AML received SC AZA (75mg/m2 QDx7d of a 28d cycle) for 1 cycle, then oral azacitidine doses ranging from 120 to 600mg (QDx7d of a 28d cycle) in subsequent cycles. In Part 2, 86 pts received oral azacitidine in 1 of 4 extended dosing schedules: 300mg QD or 200mg BID, each for 14d or 21d of repeated 28d cycles. PK parameters were derived from plasma concentrations. The correlation between PK and PD was determined using data from pts who had received oral azacitidine 200mg BID or 300mg QD for 14d or 21d for whom PD data were available at day 15 of the first 28d cycle (C1D15). The PD endpoint was reduction in percentage of highly methylated (≥70%) loci of DNA in whole blood, assessed using Illumina's Infinium Methylation27 Bead Array.
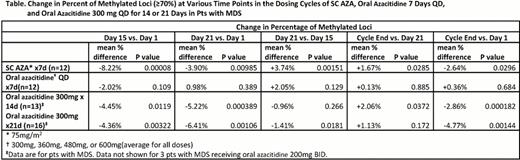
SC AZA or oral azacitidine were rapidly absorbed and reached Tmax (median [min, max]) within 0.5 hr [0.2, 1.1] and 1.0 hr [0.3, 3.6] post-dose, respectively. Mean elimination half-life was 1.5 +/− 0.7 hr and 0.6 +/− 0.2 hr for SC and oral azacitidine, respectively. No drug accumulation was noted following multiple dose administration. Compared with SC AZA, 300mg oral azacitidine QDx14d and QDx21d provided mean cumulative exposures (AUC) per cycle of 38% and 56%, respectively. A PK/PD correlation (AUC C1D1 vs. change in methylation on C1D15 compared with baseline) was observed with oral azacitidine 300mg QD or 200mg BID administered for 14d or 21d (r2=0.659, p<0.00001), with a minimum biologically effective plasma exposure of approximately 100 ng*hr/mL (Figure). Methylation was significantly reduced with SC AZA 75mg/m2 QDx7d (mean change −8.2%, p=0.00008), with maximum reduction at day 15 (Table). Methylation levels increased thereafter until cycle end (28d), although methylation did not completely return to the baseline level. Oral azacitidine (300–600 mg) QDx7d reduced the percentage of highly methylated loci at day 15, but not significantly (mean change −2.0%, p=0.109), and methylation returned to baseline level by cycle end. In contrast, extended oral azacitidine dosing at 300mg QDx14d and QDx21d in MDS pts resulted in significantly reduced methylation at day 15 and cycle end. The additional 7 days of dosing with oral azacitidine QDx21d vs. oral azacitidine QDx14d resulted in further reduction of methylation, with mean changes at day 21 of −6.4% and −5.2%, respectively. Of all azacitidine dosing regimens evaluated, including SC AZA dosing, hypomethylation between day 1 and cycle end was greatest with oral azacitidine 300mg QDx21d.
Methylation reversal was correlated with plasma AUC following oral azacitidine dosing. Consistent with the hypothesis that prolonged exposure to lower doses of DNMT inhibitors leads to improved methylation reversal, extended dosing schedules led to significantly more demethylation compared with the 7-day oral azacitidine schedule. Additionally, DNA hypomethylation was maintained at cycle end with extended oral azacitidine dosing. The oral azacitidine 300mg QDx21d dosing schedule provided the greatest level of sustained hypomethylation at cycle end of all tested dose regimens, including SC AZA. Extended dosing schedules provide the ability to achieve the PD and epigenetic activity of oral azacitidine over a longer period of time.
Laille:Celgene: Employment, Equity Ownership. Shi:Celgene: Employment, Equity Ownership. Garcia-Manero:Celgene: Research Funding, Speakers Bureau. Gore:Celgene: Consultancy, Research Funding. Kumar:Celgene: Employment, Equity Ownership. Skikne:Celgene: Employment, Equity Ownership. MacBeth:Celgene: Employment, Equity Ownership.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal