Abstract
Abstract 1197
TLE1 belongs to the Groucho/TLE family of co-repressors that act as master regulators during development affecting segmentation, neurogenesis, myogenesis, and multiple cell fate decisions. TLE1 modulate several major signaling pathways including Wnt and Notch, and specifically interacts with multiple transcription factors involved in hematopoiesis such TCF/LEF, HES1, RUNX1/AML. TLE1 has also been implicated in Crohn's disease via its interaction with NOD2, a regulator of NFkB. Our laboratory identified TLE1 as a likely AML tumor suppressor gene, commonly deleted in subgroups of AML, and others have shown its role as a tumor suppressor gene in myeloid and other hematopoietic malignancies. To better understand the role of TLE1 in hematopoiesis and leukemogenesis we created a line of Tle1 null mice.
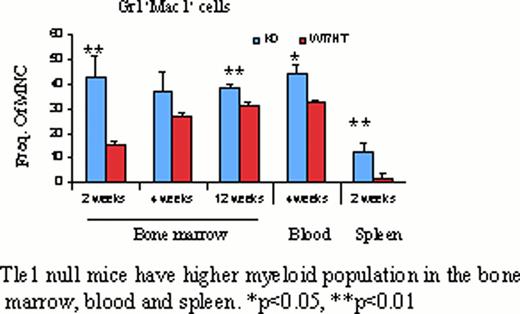
Tle1 null mice are born normally, but become progressively growth retarded by 3 days of life, with only 50% survival by 4 weeks as compared to heterozygous and wild type littermates. Abnormalities are observed in several organs systems including the hematopoietic system. We characterized the hematopoietic system in Tle1 knock out mice between two and 12 weeks of age. The bone marrow cellularity in the Tle1 knock out mice is comparable to the wild type mice at all time points examined. However, frequency of granulocyte macrophage progenitors in bone marrow mononuclear cells is significantly higher in the Tle1 knockout bone marrow compared to heterozygous and wild type mice. The proportion and number of myeloid cells as evidenced by Gr1, Mac1 expression are significantly higher in the bone marrow, spleen and blood of these knockout mice. There were significantly lower B-cells (B220+cells) in the Tle1 knockout mice compared to heterozygous and wild type.
In colony forming assays there was a trend towards higher number of CFU-GM (7.66 vs 5), p=0.07) and CFU-M (27.16 vs 12.5, p=0.05) colonies from Tle1 null bone marrow as compared to wild type bone marrow. The spleens from four week and 17 months old Tle1 knockout mice had higher frequency of Gr1-negative, Mac1-positive and F4/80 positive macrophages. We also observed a significantly higher production of the inflammatory cytokines IL6 and TNFafrom peritoneal macrophages harvested from Tle1 null mice as compared to those from wild type mice in response to TLR ligand stimulation. To investigate the potential mechanism of this inhibitory effect of TLE1 on inflammation we demonstrated that TLE1 expression is able to block the nuclear translocation of NFkB in THP1 cells in response to LPS-K12 (p<0.05).
In summary this work demonstrates that the lack of Tle1 expression biases hematopoiesis towards myeloid differentiation, a finding of potential relevance given the inactivation of TLE1 seen in subsets of myeloid malignancies. We further show that inactivation of Tle1 leads to an increase in macrophages primed to release increased inflammatory cytokines. This is notable given the recent observation that TLE1 may modulate the effects of NOD2 in the pathogenesis of Crohn's disease. These Tle1 null mice will allow the investigation of the potential role of TLE1 as a modulator of a variety of other inflammatory diseases.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal