Abstract
Abstract 1083
Drug induced immune thrombocytopenia (DITP) is a relatively common and often serious complication of treatment with many drugs. It can be extremely helpful clinically to identify the responsible agent, especially in patients taking multiple drugs. It is sometimes possible to demonstrate a drug-dependent antibody (DDAb) that binds to platelets only when drug is present but results are often negative even in patients with a history that strongly favors DITP. An important reason for this is that a drug metabolite rather than the parent drug can be the sensitizing agent. Because drugs often have many metabolites, most of which are not readily available, identification of the metabolite-specific, DDAbs can be very difficult.
Animal models for studying immune clearance of human cells have not been available because of naturally occurring xenoantibodies. However, recent studies have shown that destruction of human platelets by human antibodies can be studied in the NOD/SCID mouse, which lacks xenoantibodies (Boylan et al 2009). Metabolism of many drugs is similar in mice and humans. We have shown that mice injected with acetaminophen and naproxen produce metabolites that promote in vivo destruction of human platelets by DDAbs specific for metabolites of these drugs (Bougie et al 2010). These findings suggested that the NOD/SCID mouse might be a useful screening tool to identify putative DDAbs specific for metabolites of other drugs in patients suspected of DITP.
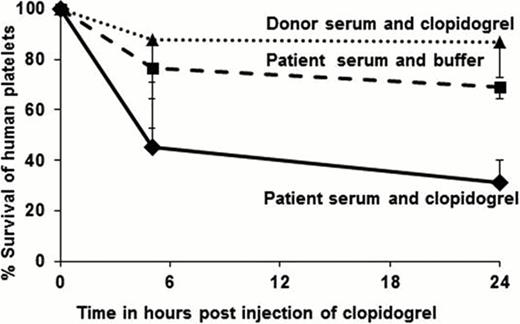
To detect metabolite-specific antibodies, mice are transfused with human platelets suspended in test serum. After collecting a baseline blood sample to define the relative number of human platelets in each mouse, the implicated drug is injected (intraperitoneal). Drug metabolites made by the mouse diffuse into the circulation and shorten the survival of human platelets if a metabolite-specific, platelet-reactive antibody is present in the test serum. For a result to be considered “positive” for DDAbs, human platelet survival should be unaltered in mice given test serum alone and normal serum plus drug and should be significantly shortened in mice given test serum plus drug (Figure).
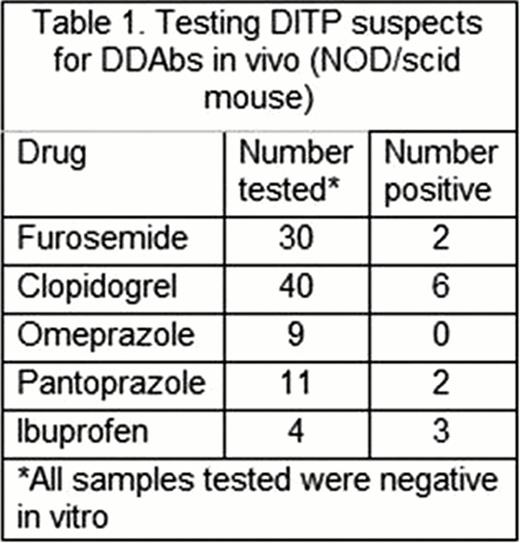
Findings made to date with 94 archived serum samples from DITP suspects exposed to 5 widely used medications are summarized in Table 1 . No platelet-reactive antibodies were detected in vitro using unmodified drug. However, 13 patient samples caused accelerated clearance of human platelets when mice were injected with the implicated drug (2 furosemide, 6 clopidogrel, 2 pantoprazole, 3 ibuprofen).
These findings suggest that DITP resulting from sensitization to drug metabolites may be much more common than has been thought and indicate that the NOD/SCID mouse model provides a highly efficient tool to screen candidate sera for metabolite-specific, platelet-reactive antibodies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal