Abstract
Abstract 1071
Hemostatic and thrombotic processes are dependent on platelets, coagulation factors, endothelial cells and hemodynamic flow. Current in vitro assays, however, only encompass one or two of these variables, rendering their results difficult to extrapolate to the in vivo setting. To that end, we have further refined our previously published endothelialized microfluidic system for studying thrombotic processes (Tsai et al, JCI, 2012) to specifically incorporate simultaneous differential flow rates spanning venous (10 s−1), capillary (100 s−1), and arterial/arteriolar (1000 s−1) flow conditions. Overall, key advantages of our system include: 1) successful integration of whole blood, an intact endothelium, and hemodynamic flow in a single microfluidic device, 2) simultaneous differential flow rates in a single experiment spanning 3 orders of magnitude, 3) use of corn trypsin inhibitor (CTI) as the sole anticoagulant, enabling calcium dependent processes to occur, and 4) minimal sample volume (1–2 mL) even for high shear conditions. We have applied our microsystem to elucidate some of the underlying mechanistic differences between venous and arterial thrombosis.
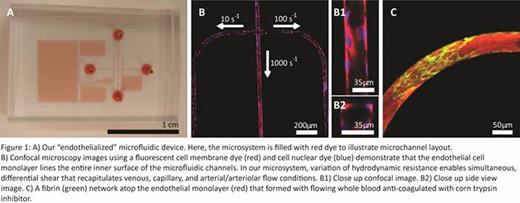
We used photolithographic and microfabrication techniques. We previously employed to develop the silicone-based microfluidic device and applied our optimized protocol to culture human endothelial cells (HUVECs) to confluency throughout the entire inner surfaces of the system (Tsai et al, JCI, 2012 and Myers et al, JoVE, 2012) (Figure 1A, 1B). Once HUVECs are successfully cultured, whole blood with 5 % v/v fluorescently-labeled fibrinogen and cell membrane dyes is flowed into the microfluidic system. Our device can then be “activated” to induce 3 different simultaneous shear conditions (10 s−1, 100 s−1 and 1000 s−1) in 3 separate endothelialized microchannels by differentially varying the hydrodynamic resistance in each microchannel. Figure 1C shows a fibrin network that is formed under flow conditions (with whole blood anticoagulated with CTI) in one of the endothelialized microchannels.
Elevated levels of prothrombin are known to increase risk of venous, but not arterial thrombosis. We applied our novel system to investigate whether differences between arterial and venous thrombosis risk depend, at least in part, on differences in blood flow/shear in those vessels. By adding 1.38 μM of prothrombin to whole blood with CTI and flowing it into our system, we observed platelet-rich and fibrin rich thrombi form and occlude the 10 s−1 and 100 s−1 microchannels, but not the 1000 s−1 microchannel. In contrast, whole blood with CTI without prothrombin yielded minimal fibrin formation on the endothelial cells with no platelet adhesion/aggregation detected in all 3 shear conditions.
Our results suggest that arterial and venous thrombosis risk is due, at least in part, to the differences in shear flow and highlights the utility of our novel endothelialized microfluidic system with simultaneous differential flow rates. Coupled with complementary in vivo experiments, our system is a powerful tool to investigate the underlying mechanisms of hemostatic and thrombotic processes involving flow.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal