Abstract
HDAC6, a major cytoplasmic deacetylase, is shown here to fine-tune the kinetics of platelet activation, a process that must be precisely regulated to ensure hemostasis after blood vessel injury while preventing pathologic thrombus formation. The discoid shape of resting platelets in the circulation is maintained by several highly acetylated microtubules organized in a marginal band. During platelet activation, microtubules undergo major reorganizations, which contribute to the shape change of activating platelets. We show that, during these activation-induced shape changes, a dramatic HDAC6-mediated tubulin deacetylation takes place, followed by microtubule reacetylation in spread platelets. In addition, although HDAC6-controlled tubulin deacetylation is not required for platelet activation, the capacity of HDAC6 to prevent tubulin hyperacetylation influences the speed of platelet spreading. These results are particularly important in view of HDAC6 inhibitors being currently used in clinical trials and represent the first example of cell signaling by lysine acetylation in platelet biology.
Introduction
Acetylation of internal lysine residues within proteins is a posttranslational modification catalyzed by acetyltransferases and removed by deacetylases.1-3 Because of its reversible character acetylation is used to regulate cellular processes.4 This has been extensively studied for nuclear processes, but there is still little knowledge concerning processes in the cytoplasm.5 Here we used blood platelets to study lysine acetylation in a strictly cytoplasmic context. Platelets are anucleated cell fragments generated by fragmentation of megakaryocytes.6 They circulate in the bloodstream as discoid particles with a diameter of 2-4 μm. On blood vessel injury, platelets become activated to ensure hemostasis. Platelet activation is composed of several consecutive and overlapping events, including adhesion to the extracellular matrix, cytoskeleton–induced shape changes, exocytosis of granules, and platelet aggregation.7 These processes must be precisely regulated to arrest bleeding in case of injury and prevent pathologic thrombus formation.8,9 When platelets are activated in vitro, they respond collectively within seconds. This rapid and synchronous response facilitates the detection of regulatory protein modifications, including acetylation.
Here we focused on the acetylation status of microtubules, which are implicated in the shape change during platelet activation. Indeed, platelets have no microtubule organizing center, yet microtubules are highly organized in resting platelets. Several tightly bundled microtubules form a ring structure, called marginal band, which is essential to maintain the discoid shape of resting platelets.10,11 Although it was shown previously that the marginal band of resting platelets is highly acetylated, the tubulin acetylation status during platelet activation had not been studied.10
We show that tubulin is deacetylated within minutes during platelet activation. Reacetylation of microtubules is observed later on in spread platelets. Taking advantage of histone deacetylase 6 knock-out (HDAC6 KO) mice,12 we demonstrate that HDAC6 is responsible for tubulin deacetylation in platelets and that tubulin deacetylation is not necessary for platelet spreading. Furthermore, we show that, in terms of platelet spreading, HDAC6 KO platelets, with hyperacetylated marginal bands, have a kinetic advantage over their wild-type (WT) counterparts.
Methods
Preparation of human PRP
Nontherapeutic buffy coats were diluted with an equal volume of PBS, centrifuged 10 minutes, 400g at room temperature, and the upper phase corresponding to the platelet-rich plasma (PRP) was collected.
Preparation of mouse PRP
Blood was collected into 100 μL 3.2% Na-citrate from anesthetized mice by cardiac puncture. Na-citrate volumes were adjusted according to recovered blood volumes. Blood with identical HDAC6 genotypes12 was pooled, centrifuged 15 minutes, 200g at room temperature to obtain the PRP.
Spreading assay
The 24-well plates with coverslips and 1 × 106 platelets/400 μL/well were centrifuged 3 minutes, 600g at room temperature. Platelets were fixed in 4% neutral formalin after different spreading times. For 0 minute time points, resting platelets were centrifuged onto glass coverslips through fixation solution.
HDAC6 activity assay
HDAC6 and control IgG immunoprecipitated complexes were incubated with an acetylated tubulin peptide (Ac-L-E-H-G-I-Q-P-D-G-Q-M-P-S-D-K(Ac)-MCA, MW1893) in reaction buffer (20mM Tris, 150mM NaCl, pH 8) for 2 hours at 37°C. Peptide mass was analyzed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry.
Results and discussion
Variations of tubulin acetylation in activated platelets
Platelets can be activated in vitro through various activation pathways, either by adding specific agonists to platelets in suspension or by letting them spread on a glass surface. To compare tubulin acetylation levels in resting and activated platelets, we performed a time course experiment of platelets spreading on glass (Figure 1A). Platelets fixed before touching the glass surface display the characteristic discoid shape of resting platelets and marginal band microtubules are highly acetylated (Figure 1A, 0 minutes). After 5 minutes of spreading, a dramatic shape change and an important decrease of acetylated tubulin are observed (Figure 1A, 5 minutes). Tubulin acetylation completely disappears after 30-60 minutes when maximal spreading is reached. Unexpectedly, microtubules become reacetylated after 2-4 hours when microtubule asters, bundles, and imperfect marginal bands reform and platelets start to retract (Figure 1A, 4 hours, ON). This process of tubulin deacetylation and reacetylation was confirmed by Western blot using platelets lysed after different incubation times on glass (Figure 1D). We next tested whether deacetylation is also induced after platelet spreading on more physiologic substrates. Like on uncoated glass, complete tubulin deacetylation is observed on spreading on fibronectin, fibrinogen, and collagen followed by reacetylation of microtubules in spread platelets (Figure 1B). In contrast, platelets are unable to spread on poly-L-lysine–coated surfaces and disc shape and microtubule acetylation remain unchanged (Figure 1B). To test whether tubulin deacetylation also occurs during platelet activation in suspension, we compared lysates of resting and aggregated platelets. Again, tubulin deacetylation is observed on platelet activation triggered by different agonists (Figure 1C). Thus, tubulin deacetylation is observed under all activation conditions tested, suggesting that deacetylation always accompanies the activation process regardless of the activation pathway.
Dynamic changes of tubulin acetylation in activated platelets. (A) Human platelets were fixed after different spreading periods (as indicated, ON indicates overnight) on glass surfaces and stained using an antiacetylated tubulin antibody (clone 6-11B-1, Sigma-Aldrich, T6793; green, top panels) or an antitubulin antibody (clone B-5-1-2, Sigma-Aldrich, T5168; green, bottom panels) detected with AlexaFluor-488 goat anti–mouse IgGs (Invitrogen; A11029). Colabeling of the actin cytoskeleton with phalloidin-rhodamine (Sigma-Aldrich, P1951; red) served as an indicator for the extent of platelet spreading. Fluorescent images were acquired using an upright microscope (Olympus BX41) equipped with a color camera DP70 using a 100× oil immersion objective and the software analySIS. (B) Human platelets were incubated for 40 minutes (left panels) or 9 hours (right panels) on glass cover slips coated with 20 μg/mL collagen (Col), fibrinogen (Fg), fibronectin (Fn), or poly-L-lysine (PLL) and blocked with 3% BSA. Platelets were then fixed and stained using an antiacetylated tubulin antibody and phalloidin-rhodamine for the actin cytoskeleton. (C) Platelets in human PRP were induced or not (no) to aggregate with 1.5mM arachidonic acid (AA), 10μM adenosine diphosphate (ADP), or 10 μg/mL collagen (Col). Aggregation was followed using an APACT 4004/LABiTec aggregometer. The PRP was then centrifuged and the platelet pellet lysed and analyzed by Western blot using an acetylated tubulin and an antitubulin antibody (inset). (D) Human platelets were allowed to spread on glass Petri dishes (6 × 107/10 mL/dish) for the indicated periods of time and then scrapped and analyzed by Western blot using an acetylated tubulin antibody. The same membrane was stained with Coomassie as a loading control. (E) Human PRP was incubated for 20 minutes on ice or at room temperature and then centrifuged. The platelet pellet was lysed and analyzed by Western blot using an acetylated tubulin and an antitubulin antibody. (F) Human platelets were incubated for 30 minutes at room temperature with 15 μg/mL nocodazole, 25μM taxol, or without drug and then either fixed in suspension (top panel) or allowed to spread on glass coverslips for 60 minutes (bottom panel). Platelets were then stained with the mouse monoclonal antiacetylated tubulin antibody and a monoclonal rabbit antitubulin antibody (clone EP1332Y, Millipore, 04-1117) detected with AlexaFluor-546 goat antirabbit IgGs (Invitrogen; A11035) as indicated. Scale bars represent 10 μm.
Dynamic changes of tubulin acetylation in activated platelets. (A) Human platelets were fixed after different spreading periods (as indicated, ON indicates overnight) on glass surfaces and stained using an antiacetylated tubulin antibody (clone 6-11B-1, Sigma-Aldrich, T6793; green, top panels) or an antitubulin antibody (clone B-5-1-2, Sigma-Aldrich, T5168; green, bottom panels) detected with AlexaFluor-488 goat anti–mouse IgGs (Invitrogen; A11029). Colabeling of the actin cytoskeleton with phalloidin-rhodamine (Sigma-Aldrich, P1951; red) served as an indicator for the extent of platelet spreading. Fluorescent images were acquired using an upright microscope (Olympus BX41) equipped with a color camera DP70 using a 100× oil immersion objective and the software analySIS. (B) Human platelets were incubated for 40 minutes (left panels) or 9 hours (right panels) on glass cover slips coated with 20 μg/mL collagen (Col), fibrinogen (Fg), fibronectin (Fn), or poly-L-lysine (PLL) and blocked with 3% BSA. Platelets were then fixed and stained using an antiacetylated tubulin antibody and phalloidin-rhodamine for the actin cytoskeleton. (C) Platelets in human PRP were induced or not (no) to aggregate with 1.5mM arachidonic acid (AA), 10μM adenosine diphosphate (ADP), or 10 μg/mL collagen (Col). Aggregation was followed using an APACT 4004/LABiTec aggregometer. The PRP was then centrifuged and the platelet pellet lysed and analyzed by Western blot using an acetylated tubulin and an antitubulin antibody (inset). (D) Human platelets were allowed to spread on glass Petri dishes (6 × 107/10 mL/dish) for the indicated periods of time and then scrapped and analyzed by Western blot using an acetylated tubulin antibody. The same membrane was stained with Coomassie as a loading control. (E) Human PRP was incubated for 20 minutes on ice or at room temperature and then centrifuged. The platelet pellet was lysed and analyzed by Western blot using an acetylated tubulin and an antitubulin antibody. (F) Human platelets were incubated for 30 minutes at room temperature with 15 μg/mL nocodazole, 25μM taxol, or without drug and then either fixed in suspension (top panel) or allowed to spread on glass coverslips for 60 minutes (bottom panel). Platelets were then stained with the mouse monoclonal antiacetylated tubulin antibody and a monoclonal rabbit antitubulin antibody (clone EP1332Y, Millipore, 04-1117) detected with AlexaFluor-546 goat antirabbit IgGs (Invitrogen; A11035) as indicated. Scale bars represent 10 μm.
Hence, a deacetylase either gets activated or gains access to its substrate during platelet activation. The latter appears to be the case because deacetylation occurs even in resting platelets as soon as microtubules are depolymerized by nocodazole or incubation at 4°C, whereas acetylation is maintained during platelet spreading when microtubules are stabilized with taxol (Figure 1E-F).
The progressive reacetylation of microtubules in spread platelets necessitates a tubulin acetyltransferase and consumes acetyl-CoA, the cofactor of the acetylation reaction. The fact that platelets are equipped with all players to produce these dynamic changes of tubulin acetylation points to a regulatory role of this posttranslational modification for cytoskeletal reorganizations.
HDAC6 mediates tubulin deacetylation in platelets
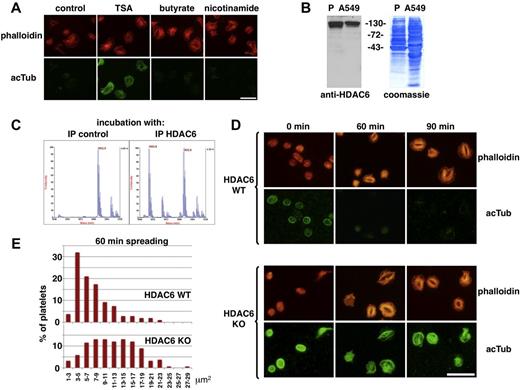
To characterize the deacetylase, spreading experiments on glass surfaces were performed in the presence of different deacetylase inhibitors. Trichostatin A (inhibiting class I/II deacetylases)13 but not Na-butyrate (inhibiting class I/II deacetylases, except HDAC6 and HDAC10)14,15 or nicotinamide (inhibiting class III deacetylases),16 inhibits tubulin deacetylation during platelet spreading (Figure 2A). This suggested that HDAC6 or HDAC10 is the enzyme responsible for tubulin deacetylation. By Western blot, we could show that HDAC6 is present in platelets (Figure 2B). Furthermore, HDAC6 immunoprecipitated from resting platelets is active in an in vitro deacetylation assay (Figure 2C). The presence of active HDAC6 in resting platelets and its known preference for tubulin dimers rather than microtubules15 corresponds to the requirements of the deacetylation mechanism described in Figure 1E and F. We therefore compared platelets from HDAC6 WT and KO mice.12 Marginal bands in resting HDAC6 KO platelets are hyperacetylated compared with WT platelets (Figure 2D). Noteworthy is that, even though spreading of mouse platelets on glass surfaces takes longer than for human platelets, rapid tubulin deacetylation parallels spreading of WT platelets similar to human platelets. During spreading of HDAC6-deficient platelets, the level of acetylated tubulin is maintained (Figure 2D), as for human platelets treated with TSA (Figure 2A). HDAC6-deficient platelets spread faster than WT platelets as observed by the larger size of KO versus WT platelets after 60 minutes of spreading (Figure 2D-E). This difference, however, was transient because WT and KO platelets occupy a similar surface area when fully spread after 90 minutes of incubation (Figure 2D).
HDAC6 mediates tubulin deacetylation during platelet activation. (A) Human platelets were fixed after 30 minutes of spreading in the absence or presence of 100 ng/mL TSA, 10mM Na-butyrate, or 5mM nicotinamide and stained using an antiacetylated tubulin antibody and phalloidin-rhodamine for the actin cytoskeleton. (B) Western blot of a human platelet lysate and a lysate of the human lung carcinoma cell line A549 (cultured in RPMI 1640/10% FCS; 70 μg/lane) revealed with an anti-HDAC6 antibody (Santa Cruz Biotechnology; sc-11420), followed by Coomassie staining of the transfer membrane. (C) Anti-HDAC6 antibodies were used for immunoprecipitation from a human platelet lysate, and unspecific IgGs were used as control. Immune complexes were incubated with an acetylated tubulin peptide (MW 1893), which was then analyzed by mass spectrometry for loss of acetylation (loss of 42 Da). Incubations with control IgGs resulted in 0% deacetylation versus 55.1% ± 2.9% deacetylation for incubations with HDAC6 immune complexes (n = 3). (D) HDAC6 WT and KO platelets in the resting state or spread for 60 and 90 minutes on glass surfaces were fixed and stained with phalloidin-rhodamine and an acetylated tubulin antibody as indicated. (E) Quantification of the surface area occupied by the actin cytoskeleton using images taken as in Figure 2D for phalloidin-rhodamine stainings after 60 minutes of spreading. The histogram represents the percentage of platelets present in different size categories as indicated on the x-axis; ∼ 150 platelets were counted for each condition of a typical experiment repeated 4 times. Scale bars represent 10 μm.
HDAC6 mediates tubulin deacetylation during platelet activation. (A) Human platelets were fixed after 30 minutes of spreading in the absence or presence of 100 ng/mL TSA, 10mM Na-butyrate, or 5mM nicotinamide and stained using an antiacetylated tubulin antibody and phalloidin-rhodamine for the actin cytoskeleton. (B) Western blot of a human platelet lysate and a lysate of the human lung carcinoma cell line A549 (cultured in RPMI 1640/10% FCS; 70 μg/lane) revealed with an anti-HDAC6 antibody (Santa Cruz Biotechnology; sc-11420), followed by Coomassie staining of the transfer membrane. (C) Anti-HDAC6 antibodies were used for immunoprecipitation from a human platelet lysate, and unspecific IgGs were used as control. Immune complexes were incubated with an acetylated tubulin peptide (MW 1893), which was then analyzed by mass spectrometry for loss of acetylation (loss of 42 Da). Incubations with control IgGs resulted in 0% deacetylation versus 55.1% ± 2.9% deacetylation for incubations with HDAC6 immune complexes (n = 3). (D) HDAC6 WT and KO platelets in the resting state or spread for 60 and 90 minutes on glass surfaces were fixed and stained with phalloidin-rhodamine and an acetylated tubulin antibody as indicated. (E) Quantification of the surface area occupied by the actin cytoskeleton using images taken as in Figure 2D for phalloidin-rhodamine stainings after 60 minutes of spreading. The histogram represents the percentage of platelets present in different size categories as indicated on the x-axis; ∼ 150 platelets were counted for each condition of a typical experiment repeated 4 times. Scale bars represent 10 μm.
These results confirm that HDAC6 is required for tubulin deacetylation during platelet activation and show that SIRT2, which deacetylates tubulin in other cells,17 cannot compensate for HDAC6 deficiency in KO platelets. Our results also demonstrate that tubulin deacetylation is not necessary for platelet spreading and show that the fine-tuning of cytoskeletal reorganizations is regulated by acetylation because the kinetics of platelet activation differ between HDAC6 WT and KO platelets. A challenging task will be now to find out which molecular interactions are directly modulated by the acetylation status of microtubules in platelets.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Joel Plumas, Sandrine Fournel, and Bernard Schweizer for arranging access to buffy coats; Corinne Albiges-Rizo for the generous gift of fibrinogen and fibronectin; Rémy Sadoul for helpful discussions; Jacques Mazzega of the optical microscopy platform (Cell imaging, CRI U823) for technical assistance; the staff of the animal care facility (PHTA, Grenoble) for excellent work; and Sophie Rousseaux for English language corrections.
The microscopy equipment was supported by the Association for Research on Cancer, MESR, and Rhone-Alpes region (CPER Exploration du vivant, imagerie biomedicale). The work in the S.K. laboratory was supported by ANR, INCa, and Association for Research on Cancer. This work was supported in part by the Science and Technology Commission of Shanghai Municipality (grant 09540700800). The work in the P.M. laboratory was supported by the Novartis Research Foundation.
Authorship
Contribution: K.S. initiated the study, designed and performed experiments, analyzed the data, and wrote the manuscript; J.W. and B.D. performed experiments and discussed the data; A.-L.V. and T.B. handled the mice; T.R. and B.P. helped with aggregation assays; X.X. discussed the data; P.M. provided the HDAC6 KO mice; and S.K. discussed experiments and data, financed the study, and contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karin Sadoul, Inserm U823, Université Joseph Fourier, Grenoble 1, Institut Albert Bonniot, F-38700 Grenoble, France; e-mail: karin.sadoul@ujf-grenoble.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal