Abstract
In patients with acute leukemia, detection of minimal residual disease (MRD) before allogeneic hematopoietic cell transplantation (HCT) correlates with risk of relapse. However, the level of MRD that is most likely to preclude cure by HCT is unclear, and the benefit of further chemotherapy to reduce MRD before HCT is unknown. In 122 children with very-high-risk acute lymphoblastic leukemia (ALL; n = 64) or acute myeloid leukemia (AML, n = 58), higher MRD levels at the time of HCT predicted a poorer survival after HCT (P = .0019); MRD was an independent prognostic factor in a multivariate analysis (P = .0035). However, the increase in risk of death associated with a similar increment of MRD was greater in ALL than in AML, suggesting that a pretransplantation reduction of leukemia burden would have a higher impact in ALL. At any given MRD level, survival rates were higher for patients treated in recent protocols: the 5-year overall survival for patients with ALL was 49% if MRD was detectable and 88% if it was not and the corresponding rates for patients with AML were 67% and 80%, respectively. Although MRD before HCT is a strong prognostic factor, its impact has diminished and should not be regarded as a contraindication for HCT.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is an established treatment for high-risk acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).1,2 Recent studies have demonstrated high success rates for HCT in children regardless of matched donor availability.3,4 With contemporary HCT protocols, the mortality related to regimen toxicity, GVHD, and infection has decreased to less than 15%,3 and the majority of patients are expected to recover from the toxicity of the procedure. However, HCT is not always curative and leukemia relapse occurs in a substantial number of patients.5-7
Persistent minimal residual disease (MRD) is a common indication for HCT in current protocols because it is associated with a high incidence of disease progression.8 A key question now is whether HCT benefits patients whose leukemia is chemoresistant to current intensive chemotherapy.9 The toxicity of HCT in patients with persistent MRD may be excessive because of preexisting organ dysfunction or infections caused by repeated attempts with intensive chemotherapy to eliminate MRD before HCT. Moreover, HCT might fail to eradicate drug-resistant leukemic cells that survive highly intensive chemotherapy regimens, a concern supported by 2 recent studies reporting high relapse rates in patients with MRD at the time of HCT.10,11 Finally, the benefit of further therapy to reduce the level of MRD before HCT and the level of MRD that would preclude the likelihood of cure after HCT are unknown.
We recently completed 2 MRD risk-directed treatment protocols that produced excellent overall treatment outcomes for pediatric patients with AML and ALL.12,13 In the present study, we sought to evaluate in this large cohort of patients not only the prognostic value of the presence of MRD at the time of HCT, but also the effect of incremental changes in the level of MRD on the probability of survival (ie, the “dose effect”) and to determine whether there is a level of MRD that is prohibitive for a curative HCT.
Methods
Patients and HCT
The MRD level was measured immediately before HCT in 122 patients who were enrolled before transplantation in 1 of the following institutional leukemia treatment protocols: AML97 (1997-2002)14 or AML02 (2002-2008)12 for AML patients or Total Therapy 13 (1991-1998),15 Total Therapy 14 (1998-1999),15 or Total Therapy 15 (2000-2007)13 for ALL patients. The indications for HCT in first complete remission have been described previously,12,13 and include 52% for persistent MRD, 26% for high-risk cytogenetics, and 21% for unfavorable subtype (eg, FAB M6, M7, or secondary AML). HCT was indicated for all relapsed AML and ALL except B-lineage ALL that relapsed > 6 months after completion of chemotherapy or isolated extramedullary relapse. The median age at the time of HCT was 11.3 years (range, 0.6-25.1). The duration of follow-up after HCT in the survivors was 6.2 years (range, 2.5-17.6) and there was no loss of follow-up.
The details of the transplantation procedures and supportive care have been described previously.3 This study was approved by the institutional review board of the St Jude Children's Research Hospital. Briefly, conditioning regimens containing total body irradiation were used in 85% of the patients. All patients with a matched donor received cyclosporine with methotrexate or mycophenolate mofetil as GVHD prophylaxis. Ex vivo T-cell depletion of the graft was performed for all patients receiving a haploidentical HCT, with additional GVHD prophylaxis using either a calcineurin inhibitor or mycophenolate mofetil. All patients received prophylaxis for Pneumocystis pneumonia. Between 1993 and 2000, all patients who were seropositive for CMV or had a CMV+ donor received ganciclovir until day 120 after HCT. Since 2001, all HCT recipients were monitored weekly by PCR assays for CMV, EBV, and adenovirus, as well as by galactomannan assay for Aspergillus. Preemptive treatments, including ganciclovir, rituximab, cidofovir, and voriconazole, respectively, were used if the surveillance tests were positive.
MRD assay
MRD was studied by flow cytometry as described previously.12,16 Briefly, mononuclear cells were enriched from BM aspirates collected in preservative-free heparin using a density step (Accu-Prep; Accurate Chemical & Scientific). Rabbit serum was added to block Fc receptors. Cells were then washed in PBS containing 0.2% BSA and 0.2% sodium azide and labeled with mAbs conjugated to FITC, PE, peridinin chlorophyll protein, or allophycocyanin, including isotype-matched nonreactive mAbs. After 10 minutes of incubation at 20°C, the 4-color stained cell preparations were washed twice in PBS plus BSA and sodium azide, fixed in 0.5% paraformaldehyde, and analyzed with a dual-laser FACSCalibur flow cytometer with CellQuest and CellQuestPro software original and updated versions (BD Biosciences). MRD results were reported as a percentage of nucleated cells expressing the leukemia-associated immunophenotypes identified at diagnosis. The MRD level was considered positive if the level was ≥ 0.1% (1 leukemic cell among 1000 BM mononucleated cells) in AML and ≥ 0.01% in ALL. High positivity was defined as ≥ 1% in AML and ≥ 0.1% in ALL. The definition of MRD positivity and estimates of MRD levels were consistent throughout the duration of the study; the markers used were periodically validated using artificial mixtures of normal and leukemic cells and side-by-side comparisons between leukemic cells and normal BM samples, including samples regenerating after chemotherapy.12,16
Statistical analysis
We used the exact test based on the Pearson χ2 statistics to compare baseline variables among patients with or without MRD at the time of HCT. The Kaplan-Meier method was used for survival estimates and the log-rank test for comparisons of survival function. Tests of heterogeneity were performed before results were combined across groups. Covariates were further investigated in a multivariate Cox proportional hazard model based on a stepwise selection strategy, and the main effect variable of MRD was held in all steps of model building. The assumption of proportional hazard was confirmed in all analyses.17 The effect of MRD was analyzed either as a categorical variable (ie, absent, low, or high level) or as a log10-transformed continuous variable (imputed conservatively as 0.01% for AML and 0.001% for ALL if the MRD test was negative). The effect of change in the level of MRD (the dose effect) on the change in the probability of survival during the observation period was analyzed and displayed using exact logistical regression models. All reported P values are 2-sided and are considered significant if < .05. Statistical analyses were performed with SAS Version 9.2 software.
Results
Type of HCT and disease status before HCT
Of the 122 patients with very high-risk AML (n = 58) or ALL (n = 64), 35 received HCTs from an HLA-identical sibling donor, 60 from a matched unrelated donor, and 27 from a haploidentical family donor. Their demographics, stratified by MRD status at the time of HCT, are listed in Table 1. Eighty-two patients were in first complete remission at the time of HCT, 29 were in second or third remission, and 11 were not in remission. As expected, persistent MRD at the time of HCT was less common in patients with leukemia in first complete remission than in those with advanced disease (P = .0001).
Patient characteristics (N = 122) by MRD status
| Characteristic, n (%) . | MRD− . | MRD+ . | P . |
|---|---|---|---|
| No. of patients | 67 (55) | 55 (45) | |
| Age, y | |||
| < 10 | 30 (45) | 24 (44) | .90 |
| > 10 | 37 (55) | 31 (56) | |
| Sex | |||
| Male | 38 (57) | 39 (71) | .11 |
| Female | 29 (43) | 16 (29) | |
| Race | |||
| White | 50 (75) | 38 (69) | .50 |
| Nonwhite | 17 (25) | 17 (31) | |
| Disease | |||
| AML | 33 (49) | 25 (45) | .68 |
| ALL | 34 (51) | 30 (55) | |
| Disease status | |||
| CR1 | 52 (77) | 30 (55) | .001 |
| CR2 | 10 (15) | 13 (24) | |
| CR3 | 5 (7) | 1 (2) | |
| No remission | 0 (0) | 11 (20) | |
| Treatment era | |||
| Early cohort | 20 (30) | 25 (45) | .08 |
| Recent cohort | 47 (70) | 30 (55) | |
| Conditioning | |||
| TBI-based | 54 (81) | 48 (87) | .32 |
| Non-TBI | 13 (19) | 7 (13) | |
| Donor | |||
| Sibling | 20 (30) | 15 (27) | .92 |
| Unrelated | 33 (49) | 27 (49) | |
| Haploidentical | 14 (21) | 13 (24) | |
| T-cell depletion | |||
| Yes | 24 (36) | 26 (47) | .20 |
| No | 43 (64) | 29 (53) | |
| CMV status | |||
| R+/D+ | 12 (18) | 15 (27) | .23 |
| R+/D− | 13 (19) | 15 (27) | |
| R−/D+ | 14 (21) | 11 (20) | |
| R−/D− | 28 (42) | 14 (25) | |
| Outcome | |||
| Alive | 48 (72) | 22 (40) | .0001 |
| Died of leukemia | 4 (6) | 16 (29) | |
| Died of TRM | 15 (22) | 17 (31) | |
| Characteristic, n (%) . | MRD− . | MRD+ . | P . |
|---|---|---|---|
| No. of patients | 67 (55) | 55 (45) | |
| Age, y | |||
| < 10 | 30 (45) | 24 (44) | .90 |
| > 10 | 37 (55) | 31 (56) | |
| Sex | |||
| Male | 38 (57) | 39 (71) | .11 |
| Female | 29 (43) | 16 (29) | |
| Race | |||
| White | 50 (75) | 38 (69) | .50 |
| Nonwhite | 17 (25) | 17 (31) | |
| Disease | |||
| AML | 33 (49) | 25 (45) | .68 |
| ALL | 34 (51) | 30 (55) | |
| Disease status | |||
| CR1 | 52 (77) | 30 (55) | .001 |
| CR2 | 10 (15) | 13 (24) | |
| CR3 | 5 (7) | 1 (2) | |
| No remission | 0 (0) | 11 (20) | |
| Treatment era | |||
| Early cohort | 20 (30) | 25 (45) | .08 |
| Recent cohort | 47 (70) | 30 (55) | |
| Conditioning | |||
| TBI-based | 54 (81) | 48 (87) | .32 |
| Non-TBI | 13 (19) | 7 (13) | |
| Donor | |||
| Sibling | 20 (30) | 15 (27) | .92 |
| Unrelated | 33 (49) | 27 (49) | |
| Haploidentical | 14 (21) | 13 (24) | |
| T-cell depletion | |||
| Yes | 24 (36) | 26 (47) | .20 |
| No | 43 (64) | 29 (53) | |
| CMV status | |||
| R+/D+ | 12 (18) | 15 (27) | .23 |
| R+/D− | 13 (19) | 15 (27) | |
| R−/D+ | 14 (21) | 11 (20) | |
| R−/D− | 28 (42) | 14 (25) | |
| Outcome | |||
| Alive | 48 (72) | 22 (40) | .0001 |
| Died of leukemia | 4 (6) | 16 (29) | |
| Died of TRM | 15 (22) | 17 (31) | |
CR indicates complete remission; early cohort, AML97 and Total Therapy 13/14; recent cohort, AML02 and Total Therapy 15; TBI, total body irradiation; R, recipient; and D, donor.
Prognostic significance of MRD
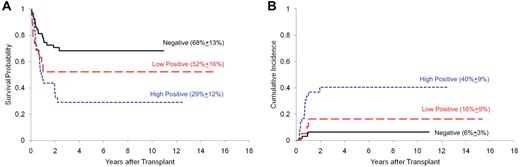
Among the 67 patients with no MRD at the time of HCT, 4 (6%) died of leukemia and 15 (22%) of transplantation-related mortality (TRM; Table 1). Death rates were higher among the 55 patients with persistent MRD: 16 (29%) patients died of leukemia and 17 (31%) of TRM (P = .0001). The 5-year cumulative incidence of relapse after HCT was 40% in the patients with high levels of MRD (defined as having ≥ 0.1% leukemia cells in ALL and ≥ 1.0% in AML), 16% among those with low level of MRD (0.01% to < 0.1% leukemia cells in ALL and 0.1% to < 1% in AML), and 6% in those with no MRD (P = .0002). Therefore, the 5-year overall survival estimate after HCT was the lowest (29%; 95% confidence interval [95% CI] 15%-45%) for patients with high levels of MRD compared with those with lower levels (52%; 95% CI, 28%-71%) or no detectable MRD (68%; 95% CI, 55%-79%; P = .0019; Figure 1). Multivariate analysis confirmed that the level of MRD, in addition to treatment era, was an independent predictor of survival (P = .0035; Table 2).
Survival and cumulative incidence of relapse after HCT stratified by MRD level. The survival (A) and relapse (B) probabilities were more favorable among patients with negative MRD than those with a low MRD level, who in turn fared better than patients with a high MRD level.
Survival and cumulative incidence of relapse after HCT stratified by MRD level. The survival (A) and relapse (B) probabilities were more favorable among patients with negative MRD than those with a low MRD level, who in turn fared better than patients with a high MRD level.
Multivariate analysis of factors affecting overall survival
| Factor . | HR . | 95% CI . | P . |
|---|---|---|---|
| MRD level* | 1.333 | 1.10-1.62 | .0035 |
| CR1 vs non-CR1 | 0.714 | 0.40-1.28 | .2571 |
| Early vs recent cohort | 3.274 | 1.76-6.10 | .0002 |
| AML vs ALL | 0.879 | 0.48-1.61 | .6759 |
| Factor . | HR . | 95% CI . | P . |
|---|---|---|---|
| MRD level* | 1.333 | 1.10-1.62 | .0035 |
| CR1 vs non-CR1 | 0.714 | 0.40-1.28 | .2571 |
| Early vs recent cohort | 3.274 | 1.76-6.10 | .0002 |
| AML vs ALL | 0.879 | 0.48-1.61 | .6759 |
CR indicates complete remission; early cohort, AML97 and Total Therapy 13/14; and recent cohort, AML02 and Total Therapy 15.
Log10 transformed.
We also performed a multivariate logistical regression analysis to allow concomitant display of the effect of treatment era and MRD (as a continuous variable) on the probability of survival. As shown in Figure 2, the higher the level of MRD at the time of HCT, the lower the survival probability. At a given level of MRD, the predicted survival was always higher for patients treated in recent protocols than for those in earlier cohorts; therefore, recent improvements in HCT have overcome, at least in part, the negative effect of MRD.
Survival probability based on the level of MRD stratified by treatment era. Probability of survival after HCT during the observation period among patients treated in the earlier era (red) and those in the recent era (blue). The confidence bands represent 95% CI limits. P < .0001 for recent versus earlier cohort and P = .0006 for MRD level.
Survival probability based on the level of MRD stratified by treatment era. Probability of survival after HCT during the observation period among patients treated in the earlier era (red) and those in the recent era (blue). The confidence bands represent 95% CI limits. P < .0001 for recent versus earlier cohort and P = .0006 for MRD level.
Differential effect of MRD in AML and ALL
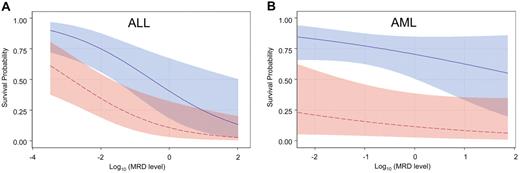
After stratifying by diagnosis (AML vs ALL), analyses were performed to estimate the potential benefit of additional chemotherapy to reduce the level of MRD before HCT. Graphs were generated using logistical regression models to display the slope of improvement in survival with each decrement of MRD level. As shown in Figure 3, the slopes were much steeper in ALL than in AML, suggesting that pretransplantation reduction of leukemia burden is potentially more beneficial in ALL.
Survival probability based on the level of MRD stratified by leukemia type and treatment era. Probability of survival during the observation period in patients with ALL (A) or AML (B) after HCT in the earlier era (red) or the recent cohorts (blue). The confidence bands represent 95% CI limits. Both ALL and AML patients treated in the recent era fared significantly better than those in the early era (P = .005 and P = .007, respectively). The impact of MRD level on survival was significant for ALL (P = .002) but not for AML (P = .18).
Survival probability based on the level of MRD stratified by leukemia type and treatment era. Probability of survival during the observation period in patients with ALL (A) or AML (B) after HCT in the earlier era (red) or the recent cohorts (blue). The confidence bands represent 95% CI limits. Both ALL and AML patients treated in the recent era fared significantly better than those in the early era (P = .005 and P = .007, respectively). The impact of MRD level on survival was significant for ALL (P = .002) but not for AML (P = .18).
To determine whether persistent MRD should be considered a contraindication for HCT because of the low chance of success, we examined the survival probability of patients with ALL or AML treated in the recent era. For patients with AML and persistent MRD (< 5% leukemia cells) at the time of HCT, the 5-year overall survival rate was 66.7% (Table 3). Even patients with AML not in remission (≥ 5% leukemia cells) had a relatively favorable survival rate (58.3%). The 5-year overall survival estimate for ALL patients with persistent MRD was 48.5% (66.7% if MRD was < 0.1% and 42.9% if it was 0.1 to < 5.0%).
Overall survival of patients in the recent cohort stratified by level of MRD
| MRD status . | AML (n = 44) . | ALL (n = 33) . | ||
|---|---|---|---|---|
| n . | 5-year estimate (95% CI) . | n . | 5-year estimate (95% CI) . | |
| < 0.01% | 27 | 80.4 (53.9-92.6) | 18 | 87.5 (58.6-96.7) |
| 0.01%- < 5.0% | 9 | 66.7 (28.2-87.8) | 13* | 48.5 (17.9-73.7) |
| ≥ 5.0% | 8 | 58.3 (18.0-84.4) | 2 | 0 |
| MRD status . | AML (n = 44) . | ALL (n = 33) . | ||
|---|---|---|---|---|
| n . | 5-year estimate (95% CI) . | n . | 5-year estimate (95% CI) . | |
| < 0.01% | 27 | 80.4 (53.9-92.6) | 18 | 87.5 (58.6-96.7) |
| 0.01%- < 5.0% | 9 | 66.7 (28.2-87.8) | 13* | 48.5 (17.9-73.7) |
| ≥ 5.0% | 8 | 58.3 (18.0-84.4) | 2 | 0 |
The 5-year survival was 66.7% (95% confidence interval [CI], 19.5%-90.4%) for the 6 patients with MRD between 0.01% and < 0.1%, and 42.9% (95% CI, 9.8%-73.4%) for the 7 patients with MRD between 0.1% and < 5%.
Discussion
In this large cohort of patients who received well-defined, MRD-based, risk-adapted chemotherapy regimens with predetermined criteria for HCT, we found that persistent MRD at the time of HCT was associated with a high rate of relapse and TRM. The poorest survival was observed in patients with a high level of MRD receiving transplantations in the early era.18 This observation is in agreement with studies by Bader et al,10 Elorza et al,19 and Jacobsohn et al,11 all of which showed a high relapse rate (50%-76%) and a poor 5-year survival rate (18%-27%) in ALL patients who had MRD > 0.01% before HCT or in AML patients who had a WT1 expression level > 0.5 relative to that of K562 cells. Close monitoring of MRD and donor chimerism after HCT is essential in these high-risk patients, because preemptive immunotherapy such as donor lymphocyte infusion and withdrawal of immunosuppression is effective in some patients.20-22
In addition to strategies after HCT to decrease risk of relapse, other efforts to improve the outcome of HCT focus on reducing the levels of MRD before HCT. However, additional intensive chemotherapy might put patients at risk for organ toxicity or life-threatening infections, and may not always reduce the leukemia burden because of enhanced resilience of leukemia cells that survive the multiagent regimens. In the present study, we used logistical regression models to examine the dose effect of MRD and to estimate the potential benefit for each decrement in MRD level. We found that the slope was much steeper in ALL than in AML, suggesting that attempts to reduce the level of MRD might be more beneficial in ALL. The exact reasons for the difference are unclear, but it is possible that AML cells are more susceptible to GVL effects.23 Because of the retrospective nature of our study and the lack of randomization, it can be argued that higher MRD levels before HCT are simply associated with a group of patients at higher risk and that they might not influence HCT outcome directly. This issue needs further investigation, but based on our results, it seems reasonable to strive toward reducing MRD levels before HCT in ALL, particularly considering the availability of newer treatment modalities such as KIR-mismatched natural killer cells, chimeric-receptor transduced effector cells, or immunotoxins, which have non-cross-resistant mechanism of actions and might reduce off-target toxicities.24-27
For patients with persistently high level of MRD despite multiple attempts at reduction therapy, a difficult issue faced by families and physicians is whether HCT is futile and should not be performed. In our recent cohort of patients with very-high-risk leukemia, patients with positive MRD (resistant to intensive reinduction attempt) had relatively high survival rates with HCT (49% for ALL and 67% for AML). Even ALL patients with high levels of MRD (0.1 to < 5.0% leukemia cells) or AML patients not in remission (≥ 5.0% leukemia cells) had a reasonably good chance of survival (43% and 58%, respectively) after HCT, suggesting that the negative effect of MRD had been partially offset by recent improvements in the procedure. TRM has decreased considerably over the years: for children and adolescents with leukemia, it was as high as 50% in the last decade, but is now less than 15%.3 Therefore, patients with a high level of MRD, in particular those with AML, should not be excluded from a potentially curative HCT. Although our patients were enrolled in treatment protocols with predefined criteria for HCT, giving high internal validity in statistical comparisons, the generalizability of our findings to patients treated with other chemotherapy regimens remains to be determined. Nevertheless, positive results of HCT have been reported recently for many high-risk patients in both the adult and pediatric populations.3-5,28,29
In conclusion, our results indicate that all pediatric patients with very-high-risk ALL or AML should be considered as candidates for HCT regardless of MRD levels during the early phase of treatment. In the present study, the era effect had a larger impact than MRD, indicating that recent improvements in the HCT procedure have offset the negative effect of MRD substantially; the impact of MRD level on survival remained significant for ALL but not for AML. Novel pre- and post-HCT treatment of MRD, such as immunotherapy or molecularly targeted therapies that are noncross-resistant to chemotherapy30-32 may further improve outcome in childhood leukemia.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank David Galloway, ELS, for assistance with manuscript editing; their clinical, laboratory, and research office colleagues for data collection; and the many patients and parents who participated in the transplantation and cellular therapy research program.
This study was supported in part by the National Institutes of Health (Cancer Center Support CORE grants P30 CA021765, U01 GM092666, and RO1 CA115422); a Center of Excellence grant from the State of Tennessee; the Assisi Foundation of Memphis; and the American Lebanese Syrian Associated Charities. C.-H.P. is an American Cancer Society Professor.
National Institutes of Health
Authorship
Contribution: W.L. designed the study, analyzed and interpreted the data, and wrote the manuscript; W.L., C.-H.P., K.G., A.S., C.H., B.M.T., M.D., A.P., D.S., J.E.R., J.T.S., S.J., H.I., R.C.R, R.H., and J.H.L provided study materials and patient information and contributed to data interpretation; E.C.-S. and D.C. performed and interpreted the MRD assays; J.Y. and D.P. performed the statistical analyses; W.L. and K.G. collected and assembled the clinical data; W.L., C.-H.P., and D.C wrote the manuscript; and all authors revised and approved the final draft of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for E.C.-S. and D.C. is Department of Paediatrics, Yong Loo Lin School of Medicine, National University of Singapore, Singapore. The current affiliation for R.H. is University Children's Hospital, Tubingen, Germany.
Correspondence: Wing Leung, MD, PhD, St Jude Children's Research Hospital, 262 Danny Thomas Pl, Memphis, TN 38105-2794; e-mail: wing.leung@stjude.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal