Abstract
B-cell antigen receptor (BCR)–mediated signaling plays a critical role in chronic lymphocytic leukemia (CLL) pathogenesis and gives an in vitro survival advantage to B cells isolated from patients with unfavorable prognostic factors. In this study, we undertook to elucidate the signaling intermediates responsible for this biologic alteration. In responding cells only, in vitro BCR engagement triggers global phosphorylation of Syk, activation of phospholipase Cγ2, and intracellular calcium mobilization, reflecting competency of BCR signaling. The calcium–calcineurin-dependent transcription factor NFAT2 is up-regulated and to some extent constitutively activated in all CLL B cells. In contrast, its DNA-binding capacity is enhanced on IgM stimulation in responding cells only. NFAT inhibition using the VIVIT peptide prevents induction of CD23 target gene and IgM-induced survival, converting responding cells to unresponsive status. At the opposite, ionomycin-induced NFAT activity allows survival of nonresponding cells. These results demonstrate that the functional heterogeneity relies on variability of protein levels establishing BCR-dependent thresholds and NFAT-dependent activation. Finally, status of the BCR-NFAT pathway for each patient reveals its relevance for CLL clinical outcome and points out to BCR-NFAT intermediates as promising functional therapeutic targets.
Introduction
Despite encouraging scientific and therapeutic advances, chronic lymphocytic leukemia (CLL) disease remains incurable with standard therapy, prompting the need for the development of novel therapeutic agents. CLL affects predominantly elderly people and, based on clinical and biologic annotations, the course of the disease can be classified from indolent to more aggressive subtypes.1-5 CLL is defined as an expansion of monoclonal, slowly dividing CD5+/CD20+ B lymphocytes. An important prognosis factor for CLL patients is the mutational status of immunoglobulin (Ig) variable heavy chain genes (IGHV) constituting the IgM B-cell antigen receptor (BCR). Considerable amounts of data have shown that antigen-driven signals are involved in the pathogenesis and progression of CLL malignancies.6-9 Although CLL cells accumulate and are resistant to cell death in vivo, they rapidly become apoptotic during in vitro culture. Interestingly, several CLL cells evade apoptosis in vitro through an enhanced survival response after BCR stimulation.6,7,9,10 After BCR triggering, which is mediated by antigen binding to cell surface Igs, prolonged activation of the MEK-ERK and PI3K-AKT pathways, as well as efficient degradation of I]kappa]B and activation of NF-κB have been associated with the induction of antiapoptotic and survival signals.11-13 These molecular events lead to important changes in gene transcription and subsequent B-cell fate specification.7,14 In addition, sustained BCR engagement promotes increased metabolic activity, allowing some restricted G1 cell-cycle progression.6,15
Most CLL cells characteristically display lower levels of surface IgM and IgD compared with normal B cells.1,2,15 Low BCR surface expression is partly explained by an inefficient assembly, trafficking, or both of Igs that are noncovalently bound to CD79a and CD79b.16 The tyrosine kinase Syk has been shown to function downstream of the BCR complex in CLL B cells.8,17 Inhibition of Syk expression or activity induces apoptosis of CLL cells both in vitro and in vivo, suggesting a prosurvival role for the kinase.18-22 Zap70, the second member of the Syk family, is considered a surrogate marker for unmutated IGHV gene expression and probably facilitates BCR signaling in CLL cells independently from its tyrosine kinase activity.23,24 Effectors downstream of Syk include, among others, phospholipase Cγ2 (PLCγ2) that is responsible for mobilization of intracellular pools of calcium.14,25 In CLL B cells, BCR ligation induces heterogeneous responses in terms of PLCγ2 phosphorylation and intracellular calcium mobilization.15,26 In turn, sustained calcium uptake activates the serine and threonine phosphatase calcineurin. Once active, calcineurin promotes dephosphorylation and nuclear translocation of the nuclear factor of activated T cells (NFAT) family of transcription factors, which cooperate with other factors to promote transcriptional regulation of numerous survival genes in lymphocytes.27,28 Although nuclear NFAT1 may present as a hallmark of unstimulated CLL B cells, the molecular events that lead to activation of NFATs and cell survival remain to be determined.29
Based on the heterogeneous response of CLL B cells in terms of in vitro cell survival on BCR ligation, we demonstrated previously that the responsiveness allows the distinction between 2 “responder” and “nonresponder” groups of patients. The responder group, characterized by cells with an elevated response in terms of transcriptional targets after sustained BCR stimulation, includes patients with unfavorable prognostic factors.6
We show in the present study that the levels of cell surface IgM and intracellular Syk, Zap70 proteins, or a combination mandate a critical threshold to promote BCR signaling in CLL B cells. Moreover, BCR competency is dependent on global phosphorylation of Syk but not Zap70, PLCγ2 activation, and intracellular Ca2+ mobilization in CLL B cells from the responder cases. Importantly, endogenous NFAT2 that is overexpressed and binds DNA in all unstimulated CLL B cells is further activated after sustained BCR stimulation in “responding” B cells only. Treatment of CLL B cells with the VIVIT peptide inhibitor antagonized the BCR-dependent stimulation of NFAT2 and blocked cell survival. The relevance of this activating pathway is demonstrated through measurement of progression-free survival (PFS) and overall survival (OS) in CLL patients. Together, our data show that targeting BCR-mediated pathways and its downstream intermediates may be a promising therapeutic approach in CLL.
Methods
CLL B-cell purification
After informed consent and in accordance with the Declaration of Helsinki, peripheral blood samples were obtained from patients fulfilling diagnostic and immunophenotypic criteria for CLL (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) at the Service d'Hématologie Biologique, Avicenne Hospital, Bobigny. B cells were purified by negative selection using RosetteSep Human B Cell Enrichment Cocktail (StemCell Technologies) following the manufacturer's instructions, and purity was verified as described previously.6 Peripheral blood mononuclear cells (PBMCs) were isolated on density gradient and further analyzed either freshly or after viable thawing from liquid nitrogen storage in fetal bovine serum (FBS; PAA Laboratories) and 10% DMSO (Sigma-Aldrich). Jurkat cell lines were grown in RPMI 1640 supplemented with 10% FBS and 1% penicillin-streptomycin (PAA Laboratories).
Metabolic activity
Two million CLL cells (purified B cells or PBMCs) were cultured for 72 hours in 24-well plates coated or not with rabbit anti-IgM antibody (10 μg/well; Jackson ImmunoResearch Laboratories). Metabolic activity was determined using CellTiter 96AQueous One Solution Cell Proliferation Assay (Promega) according to the manufacturer's instructions. Metabolic fold increase ([percentage of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium], inner salt [MTS]) was determined as follows: [(absorbance stimulated t = 72 hours) – (absorbance unstimulated t = 72 hours)]/(absorbance unstimulated t = 72 hours) × 100; MTS values greater than or equal to 25% and less than 25% defined the responder and nonresponder CLL groups, respectively.6 NFAT peptide inhibitor (11 R-VIVIT; Calbiochem) was added to cell culture media 45 minutes before IgM stimulation at final concentrations of 2 or 5μM; ionomycine (Sigma-Aldrich) was incubated for 30 minutes at final concentrations of 1, 2, or 5μM.
Immunoprecipitation and immunoblotting
Purified B cells (1 × 107) stimulated or not were lysed in 0.5% nonidet P-40 lysis buffer (50mM Tris-HCl, pH 7.4, 150mM sodium chloride, and 1mM EDTA). Immunoprecipitations were carried out using monoclonal anti-phosphotyrosine (4G10 Platinium; Millipore) or anti–human Syk (clone 4D10; BD Biosciences Pharmingen) monoclonal antibodies (mAb). After adsorption of the complex on protein G-Sepharose, beads were washed 3 times with 0.1% nonidet P-40 lysis buffer and subjected to immunodetection with the indicated antibodies (anti-Syk and -Zap70 mAbs; BD Biosciences Pharmingen). Detection was achieved using chemiluminescence (Pierce Chemical).
Flow cytometric assays
Membrane staining.
Expression of cell surface molecules was analyzed by flow cytometry, using the following mAbs: anti-CD20/APC-Cy7, CD19/APC-Cy7, CD5/PerCP-Cy5.5, CD3/PE-Cy7, CD23/PE, CD71/FITC (BD Biosciences), IgM/RPE (Dako), or relevant isotypic controls (BD Biosciences and Dako). In brief, fresh blood samples free of red blood cells, thawed PBMCs, or purified B cells were incubated in RPMI 1640 (PAA Laboratories) in the presence or not of soluble AffiniPure F(ab′)2 fragment goat anti–human IgM (10 μg/mL/106 cells; Southern Biotechnology) for 10 minutes. Cells were further labeled in 1 × phosphate-buffered saline (PBS) containing saturating concentrations of mAbs for 20 minutes, washed twice, and then analyzed immediately or further subjected to intracellular labeling.
Phosflow assay.
Phosphorylated-Syk/Zap70 (Alexa Fluor 647 anti-pY352 Syk/Zap70 pY319) and -PLCγ2 (PE anti-PLCγ2 pY759) as well as Syk and Zap70 (FITC-conjugated anti-Syk; BD Biosciences Pharmingen and R-PE anti-Zap70; Southern Biotechnology mAbs) were analyzed simultaneously using Lyse/Fix Buffer and Perm Buffer I (BD Biosciences), according to the manufacturer's protocols. Data were acquired and analyzed with a BD FACSCanto II analyzer and DIVA software (BD Biosciences).
Detection of intracellular calcium.
One million CLL B cells were washed in Cell Loading Buffer (1mM calcium, 1mM magnesium, and 1% FBS) and incubated with 2μM calcium indicator (fluo-4 AM; Invitrogen) for 30 minutes at 37°C in the dark. After further washing, cells were incubated in Cell Loading Buffer for 30 minutes at 37°C in the dark and finally analyzed by flow cytometry (BD FACSCanto II and DIVA software) for 300 seconds (stimulation was performed with 10 μg of α-IgM/mL/106 cells at t = 60 seconds and 5μM ionomycin at t = 290 seconds).
Immunofluorescence staining
Unstimulated or IgM-stimulated B cells were seeded on polylysine-coated coverslips for 30 minutes at 37°C and eventually stored at −20°C. Cells were fixed in PBS containing 4% paraformaldehyde for 10 minutes and permeabilized in PBS containing 0.1% Triton X-100 (Sigma-Aldrich) for 10 minutes. After incubation for 45 minutes in blocking solution (PBS and 5% skimmed milk), anti-NFAT2 Ab (1:500; Ozyme) was applied at room temperature for 1 hour. Coverslips were further washed in PBS and incubated with Alexa 488–coupled secondary Ab (1:1000; Invitrogen) for 1 hour at room temperature. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (Sigma-Aldrich) before slide mounting with Mowiol (Sigma-Aldrich).
RT-PCR
Total RNAs from CLL patient or healthy donor B cells were purified (QIAGEN) and quantified by spectrophotometry. cDNAs were synthesized using SuperScript II Reverse Transcriptase (Invitrogen). Amplification was performed in triplicates using SYBR Green PCR Mastermix and the appropriate primers (supplemental Methods; 10 μM, Applied Biosystems) using a 7000 SDS thermal cycler (Applied Biosystems).
ELISA for NFAT2 DNA-binding activity
NFAT2 DNA-binding capacity was measured using TransAM NFATc1 kit (Active Motif) according to the manufacturer's instructions (supplemental Methods).
Data analysis and statistics
Values are presented as mean ± SEM. Statistical significance was determined using the Mann-Whitney test as appropriate. P values of less than .05 were considered statistically significant. A 2-tailed unpaired Student t test was used to compare individual groups. A level of P less than .05 was considered significant. Statistical analyses were performed using GraphPad software. Distributions of PFS and OS were estimated by the Kaplan-Meier method. Log-rank test was performed to test the difference of survival between groups.
Results
BCR triggering with anti-IgM immobilized antibodies promotes a heterogeneous cell survival response that allowed us classifying CLL patients into 2 responder and nonresponder groups. Although responder CLL B cells exhibited increased metabolic activity, nonresponder cells showed no significant enhancement.6 Assessment of the metabolic activity of CLL cells on BCR stimulation (% MTS) on a cohort of 77 untreated CLL patients lead to their sorting into 2 subgroups: 37 nonresponder and 40 responder patients (supplemental Table 1).
We observed previously an elevated transcriptional response on BCR stimulation in CLL cells isolated from responder patients only. To dissect this functional heterogeneity between the 2 groups of patients, we examined whether B-cell survival might result from differential expression, activity, or localization of signaling effectors.
Membrane IgM, but not CD20 coreceptor, has higher levels of expression in responding B cells
Low levels of surface IgM are the hallmark of CLL B cells.16 We analyzed whether cell surface IgM levels might diverge between nonresponder and responder cases. Relative IgM surface expression was determined using flow cytometry on CD5+/CD19+ B cells isolated from nonresponder (n = 20) and responder (n = 20) patients and compared with non-CLL B cells (control; n = 11). Despite the expected overall lower membrane expression in CLL B lymphocytes compared with control B cells, leukemic B cells from responder cases exhibited significantly higher IgM levels than those from nonresponder cases (P = .0037; Figure 1A and supplemental Figure 1A). In contrast, no significant difference was observed for the CD20 marker that participates to and modulates the BCR signaling complex, between nonresponder (n = 20) and responder (n = 20) groups (Figure 1B and supplemental Figure 1B). These results demonstrate a variation in the antigen-binding components of the complex, arguing for a critical level of signaling capacity in CLL B lymphocytes.
Cell surface IgM levels as well as expression and global phosphorylation levels of Syk reflect CLL B-cell responsiveness. Presence of surface IgM (A), CD20 (B), or Syk (C) and Zap70 (D) kinase levels was assayed on normal (control) and CLL B cells using flow cytometry analysis. (A) Cell surface IgM levels were calculated and graphed relative to control isotype labeling for 11 controls, 20 nonresponder (NR) samples, and 20 responder (R) samples. MFI indicates mean fluorescence intensity. (B-D) Cell surface CD20 expression on 20 NR and 20 R patients and intracellular Syk and Zap70 on 22 NR and 24 R cases were evaluated and graphed relative to control B cells. Means (± SEM) and significant P values are indicated (ns denotes not significant). (E) Anti-phosphotyrosine immunoprecipitation ([IP]; α-pY) was performed on cellular extracts of NR (n = 6) and R (n = 6) CLL B cells. Cells were either left unstimulated (−) or stimulated (+) with anti-IgM (α-IgM) antibody. Precipitates were analyzed with anti-Syk or anti-Zap70 antibodies, allowing detection of pSyk and pZap70. Total extracts are analyzed as a control for Syk and Zap70 expression. Fold increase is calculated as a ratio between stimulated and unstimulated levels.
Cell surface IgM levels as well as expression and global phosphorylation levels of Syk reflect CLL B-cell responsiveness. Presence of surface IgM (A), CD20 (B), or Syk (C) and Zap70 (D) kinase levels was assayed on normal (control) and CLL B cells using flow cytometry analysis. (A) Cell surface IgM levels were calculated and graphed relative to control isotype labeling for 11 controls, 20 nonresponder (NR) samples, and 20 responder (R) samples. MFI indicates mean fluorescence intensity. (B-D) Cell surface CD20 expression on 20 NR and 20 R patients and intracellular Syk and Zap70 on 22 NR and 24 R cases were evaluated and graphed relative to control B cells. Means (± SEM) and significant P values are indicated (ns denotes not significant). (E) Anti-phosphotyrosine immunoprecipitation ([IP]; α-pY) was performed on cellular extracts of NR (n = 6) and R (n = 6) CLL B cells. Cells were either left unstimulated (−) or stimulated (+) with anti-IgM (α-IgM) antibody. Precipitates were analyzed with anti-Syk or anti-Zap70 antibodies, allowing detection of pSyk and pZap70. Total extracts are analyzed as a control for Syk and Zap70 expression. Fold increase is calculated as a ratio between stimulated and unstimulated levels.
Syk and Zap70 levels of expression are higher in responding CLL B cells
On triggering, BCR complex-driven signals propagate through intracellular protein tyrosine kinase of the Syk family. Therefore, Syk and Zap70 protein expressions were analyzed in CLL B cells and compared with those of normal B cells using flow cytometry. Consistent with previously published results,30 Syk protein levels were more abundant in most of CLL B cells compared with normal B lymphocytes (Figure 1C and supplemental Figure 2A). Interestingly, significantly higher levels of Syk were detected in the responding B cells compared with nonresponding cells (P = .02; Figure 1C). FISH analysis confirmed that the overexpression of Syk protein was not because of genetic amplification of SYK gene (supplemental Figure 2B). As already reported,6 Zap70 was detected more in responding cells respective to nonresponding cells and normal B lymphocytes (P = .004; Figure 1D). Together, these results suggested that high IgM, Syk, and Zap70 protein contents might contribute to reach a critical responsiveness threshold on BCR engagement in responding CLL B cells.
Global Syk phosphorylation reflects activation of the responding CLL B cells
We next investigated the constitutive and stimulation-dependent phosphorylation of Syk family members in nonresponding and responding B cell lysates (Figure 1E). Higher levels of constitutive phospho-Syk were detected in responding cells compared with nonresponding cells. Furthermore, although phospho-Syk levels remained low in nonresponding cells on IgM stimulation, increase of phospho-Syk was systematically detected in responding cells. Despite the presence of a substantial amount of Zap70 in some extracts, we did not observe any constitutive or IgM-induced Zap70 phosphorylation. Together, the differences in Syk expression and phosphorylation status in our patient samples are not only reflective of constitutive differences in BCR activation but also of a differential signaling capacity between responding and nonresponding leukemic cells.
Because several tyrosine residues of Syk are targeted for phosphorylation on BCR stimulation, we next examined the phosphorylation status of tyrosine 352 (Y352) by immunoblotting. Although the phosphorylation of Y352 was higher in responding CLL B cells compared with nonresponding cells, enhanced phosphorylation of this particular residue was not observed on IgM triggering for neither of them (supplemental Figure 2C). This result was confirmed by flow cytometry on a larger cohort of patients using the same anti-pY352 Syk antibody (supplemental Figure 2D). Although these results contrast with the global phosphorylation pattern of Syk, they argue for the functional implication of other conserved residues in the signaling capacity of the responding CLL B cells.
Together, these data further suggest a smallest possible requirement for membrane initiators, and early intermediates, to reach threshold levels of stimulatory signals. Cells reaching such levels only might have the capacity to respond on BCR stimulation.
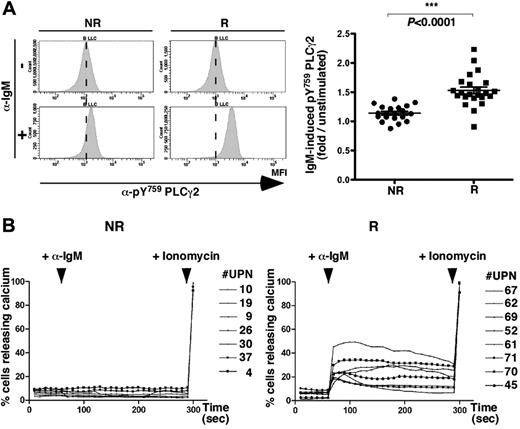
BCR allows phosphorylation of PLCγ2 and calcium mobilization in responding CLL B cells
We next evaluated whether IgM-induced activation also might lead to a differential increase of PLCγ2 phosphorylation. Using Phosflow cytometry analysis (as shown for 2 representative samples in Figure 2A left), anti-IgM stimulation led to considerable increase in pY759 PLCγ2 levels in responding cells compared with unstimulated cells but to minor extent in nonresponding cells. Mean fluorescence intensities were collected from a series of leukemic B cells, and anti-IgM–induced PLCγ2 phosphorylation was calculated for each sample and graphed. Results from a larger series of patients confirmed that PLCγ2 exhibited higher phosphorylation rates in CLL B-cell samples from the responder group compared with the nonresponder group (Figure 2A right), demonstrating that PLCγ2 was differentially activated between the 2 groups of patients.
BCR triggering promotes PLCγ2 phosphorylation and calcium mobilization in responding CLL B cells. (A) Phospho-Y759-PLCγ2 levels were analyzed using a specific PE-coupled antibody in CLL B cells (CD20+/CD5+/CD3−) by flow cytometry analysis. Histogram plots show pY759 PLCγ2 MFI in unstimulated (α-IgM−, top) and stimulated (α-IgM+, bottom) B cells from 1 representative patient for each CLL group (NR, left; R, right). Phospho-PLCγ2 was measured for 43 samples after 10 minutes of incubation with anti-IgM antibody in NR (n = 20, black dots) and R (n = 23, black squares) cases, calculated as fold induction over unstimulated cells and graphed. (B) Calcium release curves established in NR (n = 7, left) and R (n = 8, right) cases. After 60-second stabilization, CLL B cells were stimulated with anti-IgM antibody (+ α-IgM) followed at 290 seconds by ionomycin addition (+ ionomycin).
BCR triggering promotes PLCγ2 phosphorylation and calcium mobilization in responding CLL B cells. (A) Phospho-Y759-PLCγ2 levels were analyzed using a specific PE-coupled antibody in CLL B cells (CD20+/CD5+/CD3−) by flow cytometry analysis. Histogram plots show pY759 PLCγ2 MFI in unstimulated (α-IgM−, top) and stimulated (α-IgM+, bottom) B cells from 1 representative patient for each CLL group (NR, left; R, right). Phospho-PLCγ2 was measured for 43 samples after 10 minutes of incubation with anti-IgM antibody in NR (n = 20, black dots) and R (n = 23, black squares) cases, calculated as fold induction over unstimulated cells and graphed. (B) Calcium release curves established in NR (n = 7, left) and R (n = 8, right) cases. After 60-second stabilization, CLL B cells were stimulated with anti-IgM antibody (+ α-IgM) followed at 290 seconds by ionomycin addition (+ ionomycin).
To delineate the contribution of the BCR-mediated activation on Ca2+ release, we next measured intracellular Ca2+ uptake in both groups of patients. Anti-IgM stimulation did not lead to a significant release of calcium in nonresponding cells but led to efficient uptake of calcium in the responding cells (Figure 2B). Leukemic B cells from both groups showed increased levels of calcium in response to the ionophore ionomycin, demonstrating that the calcium pathway in nonresponding CLL B cells was not altered. Therefore, rapid PLCγ2 phosphorylation and calcium uptake on BCR stimulation could serve as potential biomarkers to discriminate CLL patient groups.
NFAT2 is constitutively overexpressed and to some extent localizes into the nucleus of CLL B cells
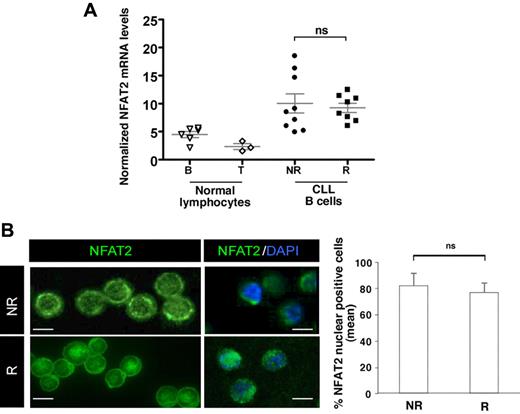
Studies have shown that the BCR/PLCγ2-calcium signaling cascade leads to the subsequent activation of NFAT transcription factors. Although NFAT1 is predominantly expressed in resting T cells, NFAT2 is induced under the positive control of NFAT1 in response to stimulation.31,32 We first evaluated by RT-PCR the transcript levels of NFAT1 and NFAT2 isoforms in CLL cells. Interestingly, both NFAT2 and NFAT1 transcript levels were higher in CLL B cells compared with normal B lymphocytes (Figure 3A and supplemental Figure 3A, respectively). NFAT1 levels in CLL B cells were comparable with those detected in T lymphocytes with even higher levels in nonresponding B cells. Despite the heterogeneous NFAT2 transcript levels in nonresponding cells compared with responding cells, quantification of mRNA amounts showed no significant difference between the 2 groups of CLL B cells.
NFAT2 is overexpressed and to some extent localized into the nucleus of CLL B cells. (A) Relative NFAT2 transcript levels were assessed by quantitative RT-PCR from freshly isolated normal blood peripheral B and T lymphocytes (normal lymphocytes B and T; n = 6 and n = 3, respectively) and from CLL B cells from NR and R cases (n = 17). NFAT levels were normalized to Abelson expression. (B) Freshly purified CLL B cells (n = 10) were immunostained with anti-NFAT2 antibody. Nuclei were counterstained with 4,6-diamidino-2-phenylindole. One representative case of each CLL group (NR and R) is presented. Images were acquired using confocal laser-scanning microscope (TCS SP2; Leica). Scale bars represent 5 μm. Single optical sections were obtained with high numerical aperture lens (63 × 2.8 NA) to determine the percentage of NFAT2-positive nuclei in NR and R cells and graphed on the right histogram; an average of 90 cells/sample (6 samples) were analyzed. No statistical difference was observed (ns).
NFAT2 is overexpressed and to some extent localized into the nucleus of CLL B cells. (A) Relative NFAT2 transcript levels were assessed by quantitative RT-PCR from freshly isolated normal blood peripheral B and T lymphocytes (normal lymphocytes B and T; n = 6 and n = 3, respectively) and from CLL B cells from NR and R cases (n = 17). NFAT levels were normalized to Abelson expression. (B) Freshly purified CLL B cells (n = 10) were immunostained with anti-NFAT2 antibody. Nuclei were counterstained with 4,6-diamidino-2-phenylindole. One representative case of each CLL group (NR and R) is presented. Images were acquired using confocal laser-scanning microscope (TCS SP2; Leica). Scale bars represent 5 μm. Single optical sections were obtained with high numerical aperture lens (63 × 2.8 NA) to determine the percentage of NFAT2-positive nuclei in NR and R cells and graphed on the right histogram; an average of 90 cells/sample (6 samples) were analyzed. No statistical difference was observed (ns).
Because NFAT2 appears as a major regulator of cell death inhibition in B cells, we focused our studies on this highly expressed factor NFAT2. Interphase and metaphase FISH analysis showed neither translocation breakpoints nor amplifications (supplemental Figure 3B), indicating that chromosomal alterations are unlikely at the origin of the higher NFAT2 mRNA levels of expression in CLL B cells.
We next determined whether NFAT2 mRNA overexpression and constitutive activation of the cells might lead to the deregulation of NFAT2 protein localization. The subcellular distribution of NFAT2 proteins in CLL B cells from both groups of patients was analyzed and compared with normal B cells using confocal microscopy. Although cytoplasmic NFAT2 only translocated into the nucleus on IgM stimulation or ionomycin treatment in normal B cells (supplemental Figure 4A), NFAT2 was detected in both the cytoplasm and the nucleus of unstimulated CLL B cells (Figure 3B left). Furthermore, quantification of NFAT2 nuclear staining on larger panels of CLL B cells showed no significant difference between nonresponder and responder patients (Figure 3B right).
Together, these data show that NFAT2 was up-regulated and partially translocated into the nucleus in unstimulated B cells from both patient groups, suggesting some constitutive activation of NFAT2 in CLL B cells.
On BCR ligation, NFAT2 is differentially activated in responding and nonresponding cells
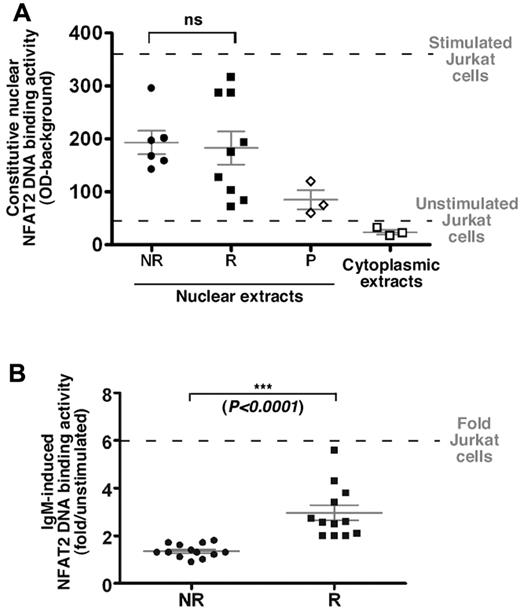
NFAT2 DNA-binding capacity was investigated in responding and nonresponding cells using an ELISA-derived assay. Although DNA binding activity of nuclear NFAT2 in our patient samples was quite heterogeneous within both groups, no significant difference was observed between responding and nonresponding cells, consistent with our immunofluorescence data (Figures 4A and 3B, respectively).
On BCR ligation, NFAT2 is differentially activated in B cells from both groups of patients. (A) Nuclear or cytoplasmic extracts from freshly isolated B cells from R (n = 9) and NR (n = 6) cases and from unstimulated or CD3+/CD28+-stimulated Jurkat cell line (horizontal dashed lines) were analyzed for their NFAT2 DNA-binding ability using an ELISA-derived assay. Competition with a specific oligonucleotide was used to ensure binding specificity (P, probe; n = 3). No statistical difference (ns) was observed between NR and R patient extracts. (B) Nuclear extracts from NR (n = 13) and R (n = 12) cells stimulated or not with an anti-IgM antibody (10 μg/mL/106 cells) for 18 hours were used following the same protocol as described in panel A. Results are presented as fold induction of the NFAT2 DNA-binding ability on stimulation.
On BCR ligation, NFAT2 is differentially activated in B cells from both groups of patients. (A) Nuclear or cytoplasmic extracts from freshly isolated B cells from R (n = 9) and NR (n = 6) cases and from unstimulated or CD3+/CD28+-stimulated Jurkat cell line (horizontal dashed lines) were analyzed for their NFAT2 DNA-binding ability using an ELISA-derived assay. Competition with a specific oligonucleotide was used to ensure binding specificity (P, probe; n = 3). No statistical difference (ns) was observed between NR and R patient extracts. (B) Nuclear extracts from NR (n = 13) and R (n = 12) cells stimulated or not with an anti-IgM antibody (10 μg/mL/106 cells) for 18 hours were used following the same protocol as described in panel A. Results are presented as fold induction of the NFAT2 DNA-binding ability on stimulation.
Interestingly, 12/12 responding CLL B cells showed increased NFAT2 DNA-binding activity in response to anti-IgM ligation (3.0-fold ± 0.3; Figure 4B). In contrast, elevation of NFAT DNA binding was almost negligible in 13/13 nonresponding cell extracts (1.3-fold ± 0.08) on IgM triggering.
Finally, both nonresponding and responding cells showed, with various extent, enhanced NFAT2 DNA-binding capacity on ionomycin treatment indicative of a functional pathway in both cell types (supplemental Figure 4B).
Together these data suggest that all CLL B cells might be in a preactivated stage, but BCR-mediated signaling is necessary to achieve significant transcriptional activation, as we observed in responding cells. Furthermore, our results indicate that IgM-induced NFAT2 DNA-binding activity discriminates CLL patient groups.
VIVIT peptide inhibitor blocks the BCR-NFAT–dependent pathway in responding CLL B cells
Given that NFAT transcription factors are translocated into the nucleus on calcineurin-dependent dephosphorylation, we tested the specific 11R-VIVIT peptide that effectively competes with NFATs for calcineurin binding.28
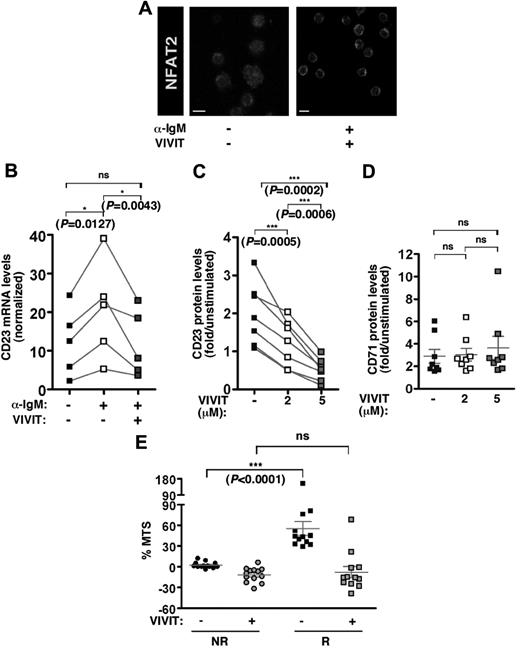
Treatment of the cells with VIVIT allowed immunodetection of NFAT2 in the cytoplasm only even on stimulation, verifying that nuclear translocation of NFAT2 was fully abolished (Figure 5A).
VIVIT peptide inhibitor blocks the BCR/NFAT-dependent pathway in responding CLL B cells. (A) NFAT2 immunofluorescence staining of CLL B cells from 1 representative R case (n = 3) stimulated or not in the presence or not of NFAT inhibitor 11-R VIVIT (5μM for 24 hours). Scale bar corresponds to 5 μm. (B) Quantitative RT-PCR analysis of CD23 transcript expression levels in freshly isolated B cells from R cases (n = 5) on stimulation with anti-IgM (10 μg/mL) in the presence or not of 11-R VIVIT (5μM). CD23 mRNA expression was normalized on ABL transcript expression in each tested sample, and results were graphed. Cell surface expression of CD23 protein (C) or CD71 protein (D) was determined using flow cytometry analysis on CLL cells from 7 (C) or 8 (D) R cases stimulated with anti-IgM (10 μg/mL) in presence of increasing concentrations (0, 2, and 5μM) of 11-R VIVIT. Levels are indicated as fold increase on stimulation. (E) Fold increase of metabolic activity (% MTS) was determined on 48-hour BCR stimulation for 13 NR and 12 R cases. B lymphocytes were incubated in the presence or not of 11-R VIVIT (5μM).
VIVIT peptide inhibitor blocks the BCR/NFAT-dependent pathway in responding CLL B cells. (A) NFAT2 immunofluorescence staining of CLL B cells from 1 representative R case (n = 3) stimulated or not in the presence or not of NFAT inhibitor 11-R VIVIT (5μM for 24 hours). Scale bar corresponds to 5 μm. (B) Quantitative RT-PCR analysis of CD23 transcript expression levels in freshly isolated B cells from R cases (n = 5) on stimulation with anti-IgM (10 μg/mL) in the presence or not of 11-R VIVIT (5μM). CD23 mRNA expression was normalized on ABL transcript expression in each tested sample, and results were graphed. Cell surface expression of CD23 protein (C) or CD71 protein (D) was determined using flow cytometry analysis on CLL cells from 7 (C) or 8 (D) R cases stimulated with anti-IgM (10 μg/mL) in presence of increasing concentrations (0, 2, and 5μM) of 11-R VIVIT. Levels are indicated as fold increase on stimulation. (E) Fold increase of metabolic activity (% MTS) was determined on 48-hour BCR stimulation for 13 NR and 12 R cases. B lymphocytes were incubated in the presence or not of 11-R VIVIT (5μM).
We next examined the effect of VIVIT treatment on CD23, a direct transcriptional target of NFAT, that is up-regulated on BCR stimulation in responding B cells only.6 RT-PCR analysis showed that BCR triggering induced CD23 transcription in responding cells. Remarkably, cotreatment of the cells with VIVIT strongly inhibited CD23 transcription in response to BCR stimulation (Figure 5B). Indeed, flow cytometry analysis revealed that CD23 protein expression also was increased on BCR stimulation in most responding CLL B-cell samples tested (n = 7). Cotreatment with increasing concentrations of VIVIT showed a dose-dependent decrease of CD23 membrane expression (Figure 5C). The specific competitive effect of VIVIT on NFAT-dependent transcription was confirmed using expression of CD71, a NFAT-independent marker of stimulated B cells (Figure 5D).
The ability of nonresponding cells to propagate signaling downstream of NFAT activation (supplemental Figure 4B) was further confirmed by a dose-dependent induction of CD23 expression on ionomycin treatment (supplemental Figure 4C). Indeed, similar induction was observed in responding cells. Together, these data demonstrated that inhibition of the calcineurin-NFAT complex, through VIVIT treatment, abolished IgM-induced NFAT transcriptional activity.
To determine whether inhibiting calcineurin-NFAT2 pathway might affect BCR-induced survival, the metabolic activity of the cells from both patient groups was evaluated in the presence of the VIVIT inhibitor (Figure 5E). Consistent with our previous results,6 we observed a much stronger increase of the metabolic activity in responding cells (n = 12) compared with nonresponding cells (n = 13; P < .0001). Interestingly, the almost 50% increase of the metabolic activity in responding cells was strongly repressed in the presence of the VIVIT peptide. In contrast, VIVIT treatment of the nonresponding cells led to a minor decrease in their metabolic activity, indicative of a very low toxicity effect on CLL B cells. Furthermore, VIVIT treatment in stimulated responding B cells resulted in metabolic levels comparable with those of the nonresponding B cells, indicating that VIVIT treatment can fully repress NFAT-driven activation pathway in responder cases. In addition, at the opposite of IgM stimulation, ionomycin treatment, that increased NFAT2 transcriptional activity of the nonresponding cells (supplemental Figure 4B), also resulted in a substantial enhancement of the metabolic activity (supplemental Figure 4D). Indeed, an ionomycin-driven activation of the metabolic activity also was observed in responding cells albeit to a lower extend than IgM stimulation. Overall, these results demonstrated that BCR-induced cell survival necessitates the calcium-NFAT signaling pathway and treatment with VIVIT altered the deleterious survival of the most responsive cells.
IgM-induced B-cell survival is relevant for CLL clinical outcome
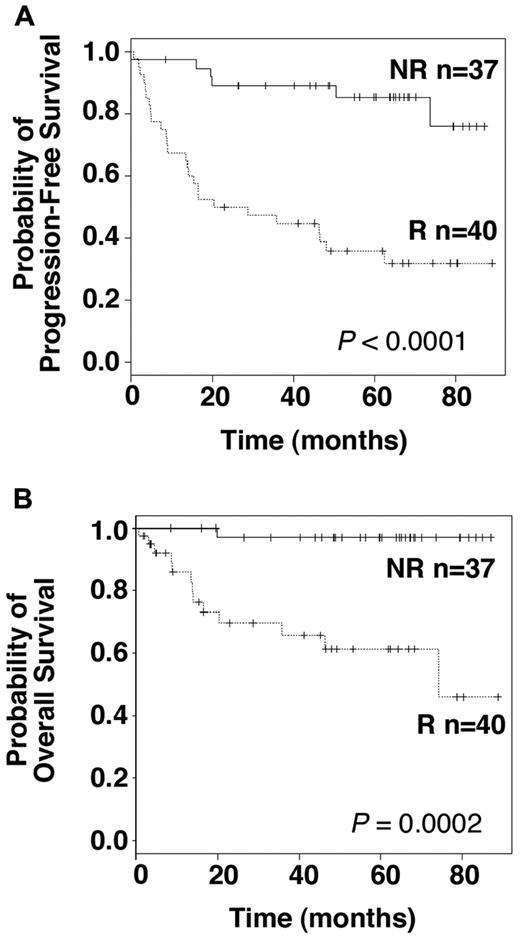
Finally, we investigated whether the ability to discriminate between responder and nonresponder patients according to their BCR-NFAT pathway activation might be of a clinical relevance. PFS and OS were assessed for the 77 untreated patients enrolled in our study, with a median follow-up of 48.6 and 60.4 months, respectively. PFS was significantly different between the responder and nonresponder group. Although median PFS was not reached for the nonresponder group, the median PFS was 20.3 months for the responder group (Figure 6A; P < .0001, log-rank test). These results demonstrate the pathophysiologic relevance of measuring IgM-induced metabolic activity in CLL B cells. Absence of BCR signaling capacity in vitro was clearly restricted to patients with stable disease, whereas IgM-induced B-cell survival advantage was associated with disease progression. Moreover, individual assessment of the early intermediates argues for the necessity of minimal amounts of these effectors to provoke cellular activation (supplemental Table 2). Even more strikingly, overall survival analysis revealed a clear difference between both groups. Median survival in the responder group was 74.2 months, whereas it was not reached in the nonresponder group. With a median follow up of 60.4 months, only 1 patient died in the nonresponder group, and from a CLL unrelated disease (Figure 6B; P = .0002, log-rank test). Therefore, IgM-induced B-cell survival advantage represents a functional predictor of the aggressive clinical course of CLL.
IgM-induced cell survival is relevant for CLL clinical outcome. Kaplan-Meier plots showing PFS (A) and OS (B) distributions in R and NR patients. Log-rank test was used to compare statistical differences in PFS and survival between both groups (P values are indicated).
IgM-induced cell survival is relevant for CLL clinical outcome. Kaplan-Meier plots showing PFS (A) and OS (B) distributions in R and NR patients. Log-rank test was used to compare statistical differences in PFS and survival between both groups (P values are indicated).
Discussion
Numerous reports, including ours, indicate an important role for BCR signaling in CLL pathogenesis and disease progression.6,7,10,33 Previously, we have demonstrated that, in vitro, BCR ligation induces a survival advantage in CLL B cells isolated from patients with unfavorable prognostic factors.6 Here, we demonstrate that the differential advantage observed in vitro translates into a striking difference for patient disease evolution. BCR unresponsiveness is clearly restricted to stable CLL cases, whereas BCR signaling capacity is associated with disease progression, shorter PFS, and shortened OS. At present, the common prognostic parameters used in the evaluation of CLL do not allow to observe such a discriminative overall survival between the 2 groups. The potential prognostic use of this novel assessment is under investigation on a larger cohort of fully annotated CLL cases by multivariate analysis with the established prognostic markers.
Further dissection of emanating signaling pathways provides evidences for a quantitative role of the initial signalosome components in the differential survival capacity of the cells between patients experiencing unfavorable or favorable clinical outcomes. Our analysis, in line with other reports, describe deregulated expression levels for various components of the BCR signaling complex in CLL B cells compared with normal B cells. In regard to this point, it has been established that low levels of BCR is a hallmark of CLL B cells because of an incorrect folding of both IgM and CD79a.3,8,15,16 However, based on the differential in vitro survival readout, B cells from the responder group express higher surface IgM levels compared with nonresponding cells. These results point out to the importance of IgM-induced signaling all along B-cell development with increasing levels required during both T1-T2 transitional stages and peritoneal differentiation.34 Furthermore, higher levels of IgM altogether with unmutated IGHV might contribute to a better recognition of multivalent antigens, providing stronger survival signals and selection in favor of the pathologic clonal expansion. The difference of surface IgM observed between nonresponding and responding cells suggests that a minimal number of receptor is necessary to reach a responsiveness threshold leading to cell survival. Absence of a significant difference for CD20 coreceptor between the 2 groups of patients is indicative of the central role held by the BCR in the threshold, and this despite the known association of this modulator with the IgM-CD79a-CD79b complex.35
Cytogenetic analysis confirmed that the transcriptional induction of SYK and NFAT2 in CLL B cells was not linked to genetic alteration.30,36 Deregulated expression might therefore be indicative of differential signaling activities in these cells in response to external BCR triggering. Of note, we observed higher levels of NFAT1, predominantly expressed in resting T cells, in the nonresponder group that exhibits low signaling capacity and metabolic activity. Interestingly, CLL B lymphocytes from responder cases expressed, on average, higher amounts of Syk and Zap70 proteins. Our results show also that a minimal amount of Syk or Zap70 is necessary to further propagate the initial signal emanating from the BCR complex and that these protein tyrosine kinases also take part in setting up signaling thresholds. Accordingly, higher expression levels of Syk and/or Zap70 might compensate lower levels of surface IgM in responding cells. Conversely, lower levels of Syk and Zap70 hinder higher membrane levels of IgM in nonresponding cells, adding value to compensatory expression levels (supplemental Table 2).
Consistent with our previous findings, constitutive tyrosine phosphorylation of Syk is observed in CLL B cells. Furthermore, it allows for a distinction between the 2 groups of patients.6,7,18,30,37 In accordance with its weak affinity for the BCR, the presence of Zap70 does not influence the basal phosphorylation of Syk. The differential constitutive phosphorylation of Syk may be because of the chronic exposure of the BCR to various soluble autoantigens and to the quantitative enhancement of both IgM and Syk expression in responding cells.7 Our discrimination between the 2 groups of patients is further supported by an increased Syk phosphorylation on IgM stimulation in B cells from responder cases only. In both constitutive and stimulatory contexts, the absence of further phosphorylation in the presence of Zap70 rather argues for an adaptor function of Zap70 in the recruitment of downstream signaling effectors.7,24,37,38 Therefore, we show that, in addition to BCR levels and structure, levels and activation of several downstream intermediates are also essential for the differential activation of the cells.
Given the stepwise activation of Syk and the alternative presence of Zap70, we also delineated the global activation status of the cells through the analysis of PLCγ2 activation and calcium flux.17,26,39,40 Differential activation was observed for both of these targets between responding and nonresponding CLL B cells. Furthermore, analysis of pY759 PLCγ2 seems to be a more reliable activation marker than other intermediates and kinases, including Syk/Zap70, Src family kinases (such as Lyn), or Btk, whose levels are all highly variable in CLL cells. Therefore, IgM-dependent PLCγ2 Y759 phosphorylation may serve as a potential biomarker of evolutivity in CLL and may be used as readout of the efficacy of Syk or Btk inhibitors. It is also tempting to speculate that specific PLCγ2 inhibitors might be developed.
Together, our study shows that the survival advantage of responding cells is the result of a balanced expression of early intermediates that establish a threshold for a cellular response. Once this threshold is reached, cells become responsive and maximize their signaling capacity by up-regulating intracellular effectors imposing a pressure for expansion through self-activation.
In terms of downstream signaling pathways, sustained engagement of the BCR results in significantly prolonged activation of Akt and ERK kinases and enhanced degradation of the NF-κB inhibitor IκB.12 BCR engagement induces activation of transcription factors, such as NFATs, that are major effectors of long-term biologic responses in B lymphocytes, including proliferation, survival, and differentiation.41 Our studies demonstrated that NFAT2 overexpression in unstimulated CLL B cells is associated with increased nuclear localization and transcriptional activity, responses that are not observed in unstimulated normal peripheral B lymphocytes. The weak constitutive NFAT2 DNA-binding activity observed in all CLL B cells might be indicative of the continuous exposure of the leukemic cells to antigens, leading to some anergic phenotype.42 These properties, together with a restricted BCR repertoire, CD5 expression, active signal transducer and activator of transcription 3, and natural IgM antibodies production are shared between CLL and murine B1a cells.1,2,7,8,30,42,43 In mice, NFAT2 genetic invalidation dramatically affects the B1a cellular compartment, whereas NFAT2 overexpression in NFAT2−/− B cells partially rescues the development and survival of these cells.43 We demonstrated that NFAT2 DNA-binding activity is induced on IgM ligation in CLL B cells from the responder group only. Thus, the active IgM-dependent signaling might allow for bypassing the anergic process in this group, but nonresponding cells could not overcome threshold levels. Accordingly, ionomycin treatment that shunts upstream molecular intermediates of the BCR signaling pathway restores induction of NFAT2 DNA-binding in nonresponding cells. However, the global lower responsiveness of these cells also argues for the requirement of upstream BCR signaling to bypass the anergic process.
To demonstrate the functional implication of NFAT2 as a biomarker in CLL B cells, we used the NFAT-specific inhibitor 11R-VIVIT, shown to be more selective than cyclosporin A and FK506, and a biomarker that can actively compete for calcineurin binding to block NFAT dephosphorylation.28 VIVIT robustly blocked nuclear translocation of NFAT on stimulation in responding CLL B cells. Remarkably, trapping NFAT2 into the cytoplasm by VIVIT treatment resulted in a clear effect on cell survival in responding CLL B cells. Inhibition of NFAT nuclear translocation led responding CLL B cells to resemble nonresponding cells. Conversely, translocation of NFAT via ionomycin treatment induced nonresponding cells to resemble responding cells. These results demonstrate the essential role of NFAT in maintaining the cell-fate balance of these cells. Consistent with the role of NFATs as transcriptional regulators, VIVIT treatment blocked the expression of CD23, a direct transcriptional target of NFAT2. Interestingly, membrane CD23 has been targeted for antibody therapy in CLL disease. This type of treatment leads to B-cell apoptosis, further supporting a role for CD23 in CLL B-cell survival.44 The self-activating loop of the responding CLL B cells might therefore be a powerful target for therapy in patients who present a progressive profile. This potential therapeutic avenue should be further investigated.
We demonstrate that nonresponding and responding CLL B cells exhibit differential signaling responses downstream of the BCR, in terms of IgM-induced global Syk phosphorylation, PLCγ2 activation, calcium release, and NFAT2 transcriptional activity. In addition, our study supports the usefulness of these various intermediates as more practical functional markers of B cells from CLL patients who have an unfavorable clinical course. In terms of therapeutic options, several intermediates of the signaling cascade might be promising targets for disease treatment. For example, the NFAT inhibitor peptide VIVIT, which does not compromise non-NFAT–mediated calcineurin signaling, abolishes cell survival response specifically generated downstream of BCR ligation.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Dr R. Balderas (BD Biosciences, US), J. F. Mathieu and F. Navarro (BD Biscoences, Europe) for providing flow cytometry reagents and antibodies directed against Syk and PLCγ2 proteins and their phosphorylated forms. BAC clones RP11-91C19 and RP11-803N2 were kindly provided by Dr S. Romana (Equipe Mixte Inserm 0210, Hôpital Necker Enfants Malades, Paris, France). They acknowledge S. Saint Georges for assistance in sample preparation and Dr L. Izzi (Montréal, QC) for critical reading of the manuscript.
This study was supported by Inserm, University Paris Nord, and grants from Ligue Nationale Contre le Cancer, Association pour la Recherche contre le Cancer, Fondation de France, and Fondation pour la Recherche Médicale. C.L.R. was the recipient of a Servier ParisBio fellowship. P.A.D. and N.C. were recipients of Fondation pour la Recherche Médicale and Société Française d'Hématologie fellowships.
Authorship
Contribution: C.L.R., P.-A.D., N.C., T.B., and M.Q. performed the experiments and analyzed the data; V.E. performed FISH experiments; R.L. shared expertise in flow cytometry experiments; C.L.R. and P.-A.D. designed the figures and wrote the manuscript with F.A.-C. and N.V.-B; V.L. and F.A.-C. provided patient samples with biologic criteria; and M.B. and V.L. performed statistical analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nadine Varin-Blank, UMR 978 Inserm-Université Paris 13, Adaptateurs de Signalisation en Hématologie, UFR-SMBH, 74 rue Marcel Cachin, 93017 Bobigny Cedex, France; e-mail: nadine.varin@inserm.fr; or Florence Ajchenbaum-Cymbalista, Laboratoire d'Hématologie Biologique, Hôpital Avicenne, 125 rue de Stalingrad, 93009 Bobigny Cedex, France; e-mail: florence.cymbalista@avc.aphp.fr.
References
Author notes
C.L.R., P.-A.D., and N.C. contributed equally to this study.

![Figure 1. Cell surface IgM levels as well as expression and global phosphorylation levels of Syk reflect CLL B-cell responsiveness. Presence of surface IgM (A), CD20 (B), or Syk (C) and Zap70 (D) kinase levels was assayed on normal (control) and CLL B cells using flow cytometry analysis. (A) Cell surface IgM levels were calculated and graphed relative to control isotype labeling for 11 controls, 20 nonresponder (NR) samples, and 20 responder (R) samples. MFI indicates mean fluorescence intensity. (B-D) Cell surface CD20 expression on 20 NR and 20 R patients and intracellular Syk and Zap70 on 22 NR and 24 R cases were evaluated and graphed relative to control B cells. Means (± SEM) and significant P values are indicated (ns denotes not significant). (E) Anti-phosphotyrosine immunoprecipitation ([IP]; α-pY) was performed on cellular extracts of NR (n = 6) and R (n = 6) CLL B cells. Cells were either left unstimulated (−) or stimulated (+) with anti-IgM (α-IgM) antibody. Precipitates were analyzed with anti-Syk or anti-Zap70 antibodies, allowing detection of pSyk and pZap70. Total extracts are analyzed as a control for Syk and Zap70 expression. Fold increase is calculated as a ratio between stimulated and unstimulated levels.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/2/10.1182_blood-2011-12-397158/4/m_zh89991293530001.jpeg?Expires=1769091601&Signature=2C9MPwDaqB~ytG8LIz5p-PNnV8cy~ksHuGnq6GVj5siT22MNvlsn3-T2WZRZDEikVfnaI1R9GtSI4jgzQA1RILVTUjhEr6tCbej7nHKdCx~Tpc62LsqRZMjLwGJDbhcjND3qBb0x1kklhquxeM5vbgPTyrvOZaFszulZMaPV5xdKI26W9syZ70z3zBVPgJx7DQNhzuYMXCbkHoDxIVqd17g77vysxx5BbR1s0jkGvUngbpgAOCDsO9MdTHGnJHUUsN3LrDXceD9wO-ZUoxTz4SE1cQ0owm7Q9NYzE2T8Z3sIRZSrsccqe5xvz1AIKdSMAXp74Eoz3-l6BrOG4xviiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal