Abstract
Hematopoietic stem cell (HSC) regulation is highly dependent on interactions with the marrow microenvironment. Controversy exists on N-cadherin's role in support of HSCs. Specifically, it is unknown whether microenvironmental N-cadherin is required for normal marrow microarchitecture and for hematopoiesis. To determine whether osteoblastic N-cadherin is required for HSC regulation, we used a genetic murine model in which deletion of Cdh2, the gene encoding N-cadherin, has been targeted to cells of the osteoblastic lineage. Targeted deletion of N-cadherin resulted in an age-dependent bone phenotype, ultimately characterized by decreased mineralized bone, but no difference in steady-state HSC numbers or function at any time tested, and normal recovery from myeloablative injury. Intermittent parathyroid hormone (PTH) treatment is well established as anabolic to bone and to increase marrow HSCs through microenvironmental interactions. Lack of osteoblastic N-cadherin did not block the bone anabolic or the HSC effects of PTH treatment. This report demonstrates that osteoblastic N-cadherin is not required for regulation of steady-state hematopoiesis, HSC response to myeloablation, or for rapid expansion of HSCs through intermittent treatment with PTH.
Introduction
The molecular signals that mediate regulatory microenvironmental osteoblastic-hematopoietic interactions provide potential therapeutic targets for hematopoietic stem cell (HSC) manipulation1 but are still largely unknown. Based on the essential role of cadherins in fate specification of germline stem cells2 and the close proximity of N-cadherin–expressing osteoblastic cells to HSCs,3-6 it was initially proposed that N-cadherin may provide HSCs with instructive interactions with their niche. However, much debate was subsequently raised by reports questioning N-cadherin expression by HSCs,7 whereas others suggested that different N-cadherin levels characterize functionally distinct HSC populations,5,8,9 which may respond to niche manipulation.10 Moreover, inducible global genetic N-cadherin deletion lacks hematopoietic defects,11 but knockdown of N-cadherin in HSCs suppresses their long-term engraftment.12 Whereas in HSCs N-cadherin, when present, is expressed at very low levels,13 osteoblastic cells express N-cadherin at several differentiation stages, both in immature and mature cells.3,14 Indeed, whereas germline deletion of the gene encoding N-cadherin (Cdh2) is embryonically lethal,15 hemizygous mice already display osteoblastic defects, having accentuated bone loss with ovariectomy.16 Moreover, recent data have suggested differential roles of N-cadherin at various stages of osteoblastic differentiation, with actions of N-cadherin both on osteogenic commitment as well as at terminal differentiation.14 In this work, our goal was to determine whether targeted deletion of Cdh2 in maturing osteoblasts alters the BM microarchitecture, HSC number, and function in homeostasis and disrupts the action of parathyroid hormone (PTH) on the skeleton and/or on hematopoietic stem and progenitor cells (HSPCs).
Methods
Mice
Col2.3-Cre mice expressing the Cre-recombinase under the control of the 2.3-kb fragment of the α1(I) collagen gene promoter were kindly provided by Dr Gerard Karsenty.17 Mating of Col2.3-Cre with Cdh2fl mice (generated in Dr Glenn L. Radice's laboratory, Thomas Jefferson University, Philadelphia, PA)18 resulted in mice carrying the Col2.3-Cre+Cadh2fl/fl genotype, which had specific inactivation of N-cadherin in cells of the osteoblastic lineage, designated as OB-NCadh for the rest of the manuscript.14 This line was maintained in the C57bl/6 background, and expression of the CD45.2 congenic marker was confirmed. Wild-type (WT) mice expressing the CD45.1 congenic marker (B6.SJL-Ptprca Pep3b/BoyJ CD45.1) were purchased from The Jackson Laboratory. Genotyping was performed as previously described.14 B6.129(Cg)-Tg(CAG-Bgeo/GFP)21Lbe/J (Z/EG) mice were also purchased from The Jackson Laboratory and were genotyped according to The Jackson Laboratory's recommendations. Mice were maintained under microisolator technique. All experiments on mice were approved by the Institutional Animal Care and Use Committee at the University of Rochester School of Medicine.
Osteoblastic cell collection
Osteoblastic cells were isolated using collagenase digestion and magnetic separation based on CD45 expression as previously described.19 Osteoblastic cells from adult mice were obtained from the long bones of the hindlimbs and forelimbs, as well as from calvaria. Osteoblastic cells from neonatal mice were obtained from calvaria.
Analysis of mRNA abundance by real-time RT-PCR
Total mRNA was extracted using the RNeasy kit (QIAGEN) according to the manufacturer's instructions. Total mRNA was then reverse-transcribed to produce cDNA using the Quantitect Reverse Transcription kit (QIAGEN). cDNA was amplified using MyiQ Single Color PCR detection systems and MyIQ Version 1.0.410 software under the following conditions: 95°C for 3 minutes followed by 40 cycles of 95°C for 15 seconds and 58°C for 30 seconds. Data were analyzed using the relative standard curve method, normalized to β-actin: β-actin, 5′ primer GCCACTGCCGCATCCTCTT and 3′ primer GGAACCGCTCGTTGCCAATAG; and Cdh2, 5′ primer ATTCAGCACCCACCTCAGTC and 3′ primer TCCGCCTCTTGAGGTAACAC.
Histology and immunohistochemistry
Harvested hindlimbs were fixed, decalcified, and processed as described.20 Histologic sections were stained with H&E to visualize morphology. Paraffin-embedded sections were cut at 4-μm thickness. All slides were deparaffinized and rehydrated to PBS (pH 7.4) and blocked in 5% normal goat serum for 60 minutes. Blocking serum was removed and 1:50 dilution of anti–N-cadherin antibody (IBL no. 18571) was applied overnight at 4°C. The biotinylated secondary antibody goat anti–rabbit at 1:200 dilution (Vector BA-1000) was then applied for 30 minutes at room temperature. The alkaline phosphatase-based ABC-AP detection system (Vector AK-5000) was applied for 30 minutes followed by Vector Red chromogen (Vector SK-4100) for 20 to 30 minutes. Slides were counterstained with fast green solution followed by 1% glacial acetic acid, dehydrated, and coverslipped with cytoseal.
Light microscopy
Histology slides were viewed at room temperature with a Bx41 upright microscope (Olympus). Objectives used were UPlan Fl 4×/0.13, UPlan Fl 20×/0.50, and UPlan FLN 60×/0.90 (Olympus). All images were obtained with a SPOT Insight 4 digital microscope camera and SPOT Version 6.7 software (SPOT).
Micro-CT analysis
The limbs were scanned on a Viva CT 40 (Scanco Medical) using a 55-kVp, 145-μA current and a 300-ms integration time with a resolution of 12.5 μm. Trabecular analysis was conducted on a 1.25-mm region 50 μm above the growth plate in the femur and a 625-μm region 50 μm below the growth plate in the tibia. Cortical analysis was conducted 4.375 mm above/below the growth plate of the femur/tibia for a distance of 375 μm.
Flow cytometric analysis
BM mononuclear cells were obtained as previously described.21 A total of 106 to 107 cells were then stained to identify Lineage−Sca1+c-kit+ (LSK) cells as previously described.21 HSPCs were identified by the phenotypic markers. Stained samples were analyzed on a LSR-II (BD Biosciences), and results were quantified using Flowjo software (TreeStar). All antibodies used for flow cytometric analysis are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Blood and spleen analysis
Blood was collected by mandibular sampling, and samples were run through the CBC-DIFF Veterinary Hematology System (HESKA) to obtain platelet, white blood cells (WBCs), and hematocrit counts. Spleens were collected in their entirety, weighed, and then mechanically disrupted to obtain a total cell count.
Competitive transplantation assay
For experiments in OB-NCadh mice and littermate controls, BM cells harvested as above for flow cytometric analysis from OB-NCadh and control (NCadhfl/fl) mice (n = 4-7 donor mice) was mixed with competitor CD45.1 marrow cells at a ratio of 1:4 (for 6-month-old mice) or 1:2 (for 1-year-old and for 2- to 3-month-old mice treated with intermittent PTH; donor/competitor) and 750 000 total cells were transplanted into each CD45.1 recipient mice (10 recipient mice per genotype). Recipient mice received a split dose of radiation of 5 Gy each separated by 24 hours. The second dose of radiation occurred 1 to 2 hours before the transplantation. For PTH-treated mice and controls, whole BM from PTH and vehicle (VEH)–treated CD45.1 C57/BL6 mice (n = 3 donor mice per treatment group) was harvested as previously described21 and mixed with competitor BM cells from CD45.2 at a ratio 1:2 (donor/competitor), and 750 000 total cells were transplanted into each CD45.2 recipient mice. The peripheral blood of transplanted mice was sampled by mandibular bleeds at times indicated to monitor engraftment. Blood was separated in a 2% solution of 5 × 105 molecular weight dextran to precipitate the RBCs. The resulting supernatant containing WBCs was analyzed by flow cytometric analysis to assess expression of congenic hematopoietic markers. For secondary transplantation, BM was harvested from competitively transplanted animals, which contained a combination of CD45.1 and CD45.2 marrow 20 weeks after primary competitive repopulation. BM from 3 animals of the same treatment group was pooled, and 750 000 cells were transplanted into irradiated CD45.2 recipient mice.
PTH injections
rPTH (1-34) was purchased from Bachem and resuspended in water to 400 μg/mL. This solution was diluted 1:100 in sterile PBS and administered intraperitoneally to WT 8- to 10-week-old C57/BL6 male mice or control and experimental mice of the ages and sex designated at 40 μg/kg 3 times daily for 10 days. Fifteen hours after the last injection, the mice were killed. The left hind limb was harvested for micro-CT analysis and histology. BM was collected from the right hind limb and used for hematopoietic analysis.
Statistical analysis
For quantitative assays, treatment groups were reported as mean plus or minus SEM. Statistical analysis was performed using the 2-tailed Student t test or 2-way ANOVA with Bonferroni Multiple Comparison posttest, when multiple comparisons to control group were made. For contingency analysis of engraftment, a threshold was set at less than or equal to 1% engraftment, 2-sample test for equality of proportions was applied, and the Fisher exact t test was used to calculate significance. Statistical significance was denoted by P ≤ .05 (Prism Version 4.01 for Windows; GraphPad Software).
Results
Genetic deletion of osteoblastic N-cadherin increases trabecular volume in adult male mice
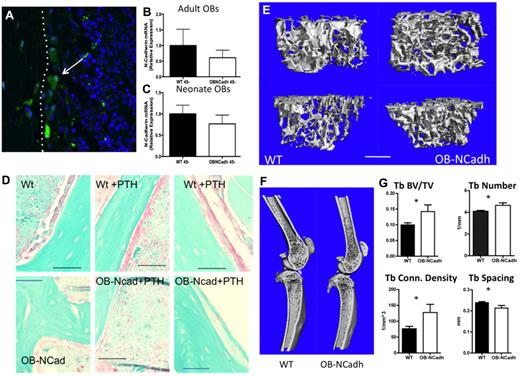
Targeted deletion of Cdh2 in osteoblastic cells did not result in any overt skeletal abnormalities. Specific expression of Cre recombinase under the control of the 2.3Col promoter was demonstrated by confocal microscopic imaging of frozen sections from 2.3Col-Cre+Z/EG+ mice (Figure 1A) consistent with previously published data.17 We determined the levels of Cdh2 gene expression in a population of cells that was previously determined to be enriched for osteoblastic cells.19 We observed a trend toward a decrease in the expression of Cdh2 in cells isolated from OB-NCadh mice compared with those isolated from WT littermate controls (Figure 1B-C). Cdh2 expression was highly variable in WT controls probably because of a lack of purity in the population of cells analyzed. This variability, coupled with the low level of Cdh2 gene expression under normal conditions, results in our inability to effectively measure a reduction in Cdh2 mRNA in OB-NCadh mice. Therefore, we used immunohistochemistry, which demonstrated a loss of N-cadherin+ cells lining the endosteal surface of bone in adult OB-NCadh mice (Figure 1D). At 2 to 3 months of age, OB-NCadh mice did not exhibit an aberrant bone phenotype compared with WT littermate controls (data not shown; see Figure 8); however, by 6 months of age, OB-NCadh mice had elevated levels of trabecular bone compared with WT littermate controls as seen in micro-CT scanning of the long bones from the hindlimbs. OB-NCadh mice had increased trabecular structures at the metaphysis of the femur and tibia (Figure 1E) that extend farther into the diaphysis than the trabecular structures in their WT littermates, as can be seen in representative micro-CT scans (Figure 1F). Quantification of micro-CT scans revealed significant increases in trabecular bone volume/total volume, trabecular number, trabecular connectivity density, and trabecular spacing in OB-NCadh mice compared with WT littermates (Figure 1G). Serum C-terminal telopeptides (CTX) levels were decreased in OB-N-Cad mice compared with WT littermates (28.86 ± 0.9835 vs 20.16 ± 2.694, P < .05, N = 3 or 4 per genotype), suggesting a decrease in bone resorption in 6-month-old mice lacking osteoblastic N-cadherin. These data reveal age-dependent changes in the trabecular architecture of mice lacking osteoblastic N-cadherin.
Lack of osteoblastic N-cadherin results in increased bony trabecular structures. (A) Representative image of a frozen section from a Col1-cre, Z/EG mouse. eGFP resulting from Cre recombination is green and highlighted by the arrow. The white dotted line indicates the endosteal surface with bone to the left and marrow to the right. (B-C) Quantitative RT-PCR analysis of N-cadherin mRNA expression. (B) Cells isolated from adult long bones. (C) Cells isolated from neonate calvaria. (D) Representative images of paraffin sections from WT (top panels) or OB-NCadh mice (bottom panels) that are untreated (left panels) or treated with PTH (middle and right panels) immunohistochemically stained for N-cadherin in which N-cadherin+ cells are pink. Images obtained using a 60× objective. (E) Representative micro-CT scans of trabecular bone at the metaphysis of the femur (top) or tibia (bottom). (F) Representative micro-CT scans of the femur and tibia. (G) Analysis of micro-CT scans showing trabecular BV/TV, trabecular number, trabecular connectivity density, and trabecular spacing. *P < .05. n = 6 to 12 male mice per group.
Lack of osteoblastic N-cadherin results in increased bony trabecular structures. (A) Representative image of a frozen section from a Col1-cre, Z/EG mouse. eGFP resulting from Cre recombination is green and highlighted by the arrow. The white dotted line indicates the endosteal surface with bone to the left and marrow to the right. (B-C) Quantitative RT-PCR analysis of N-cadherin mRNA expression. (B) Cells isolated from adult long bones. (C) Cells isolated from neonate calvaria. (D) Representative images of paraffin sections from WT (top panels) or OB-NCadh mice (bottom panels) that are untreated (left panels) or treated with PTH (middle and right panels) immunohistochemically stained for N-cadherin in which N-cadherin+ cells are pink. Images obtained using a 60× objective. (E) Representative micro-CT scans of trabecular bone at the metaphysis of the femur (top) or tibia (bottom). (F) Representative micro-CT scans of the femur and tibia. (G) Analysis of micro-CT scans showing trabecular BV/TV, trabecular number, trabecular connectivity density, and trabecular spacing. *P < .05. n = 6 to 12 male mice per group.
Genetic deletion of osteoblastic N-cadherin does not alter steady-state hematopoiesis
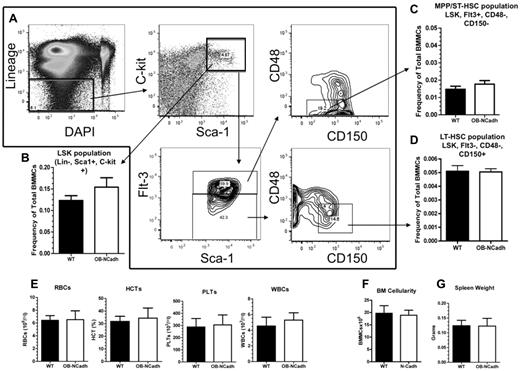
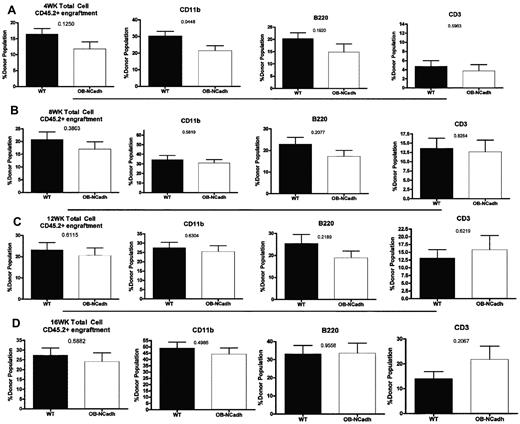
Despite the loss of osteoblastic N-cadherin and changes to the trabecular architecture of OB-NCadh mice there, were no discernible changes in steady-state hematopoiesis at 6 months of age. The frequency of phenotypically defined LSK cells (Figure 2A), a population that is enriched for HSPCs, was not significantly altered in OB-NCadh mice compared with WT littermate controls (Figure 2B). In addition, the Flt3+CD48−CD150− subpopulation of LSK cells (Figure 2A) enriched for multipotent progenitor (MPP)/short-term HSCs (ST-HSCs) and the Flt3−CD48−CD150+ subpopulation of LSK cells (Figure 2A) enriched for long-term HSCs (LT-HSCs) were also unchanged in OB-NCadh mice compared with WT littermates (Figure 2C-D). Peripheral blood cells were also not significantly different in OB-NCadh mice, including RBCs, hematocrit, platelets, and WBCs (Figure 2E). BM cellularity was unchanged (Figure 2F), and splenic weight was the same in OB-NCadh mice and WT littermate controls (Figure 2G), suggesting that extramedullary hematopoiesis in the spleen was unchanged. Functional analysis of HSCs by competitive transplantation also revealed no difference between OB-NCadh mice and WT littermate controls at 6 months of age. Peripheral blood analysis of recipient mice at 4, 8, 12, and 16 weeks revealed no difference in the engraftment of total CD45+ cells or in the engraftment of populations positive for CD11b, B220, or CD3ϵ (Figure 3). These data suggest no functional changes in ST or LT engrafting HSCs from the marrow of OB-NCadh mice compared with WT littermate controls.
Lack of osteoblastic N-cadherin does not alter steady-state hematopoiesis. (A) Representative flow cytometry plots showing the isolation of LSK, MPP/ST-HSC, and LT-HSC populations. (B) Frequency of the LSK population of cells as defined by panel A. (C) Frequency of the MPP/ST-HSC population of cells as defined by panel A. (D) Frequency of the LT-HSC population of cells as defined by panel A. (E) Peripheral blood cell counts of red blood cells, hematocrit, platelets, and WBCs. (F) Total marrow cellularity as determined by counting flushed marrow cells. (G) Spleen weight in grams. n = 6 to 12 mice per group for flow cytometric analysis, 4 to 9 per group for all other experiments.
Lack of osteoblastic N-cadherin does not alter steady-state hematopoiesis. (A) Representative flow cytometry plots showing the isolation of LSK, MPP/ST-HSC, and LT-HSC populations. (B) Frequency of the LSK population of cells as defined by panel A. (C) Frequency of the MPP/ST-HSC population of cells as defined by panel A. (D) Frequency of the LT-HSC population of cells as defined by panel A. (E) Peripheral blood cell counts of red blood cells, hematocrit, platelets, and WBCs. (F) Total marrow cellularity as determined by counting flushed marrow cells. (G) Spleen weight in grams. n = 6 to 12 mice per group for flow cytometric analysis, 4 to 9 per group for all other experiments.
Lack of osteoblastic N-cadherin does not alter numbers of functional HSCs. Analysis of the peripheral blood after competitive transplantation shows the percentage of donor cells determined by cell surface expression of CD45.2 in the total peripheral blood cell population, CD11b+ population, B220+ population, and CD3e+ population. Recipient mice were analyzed at (A) 4 weeks, (B) 8 weeks, (C) 12 weeks, and (D) 16 weeks after transplantation of donor and competitor cells. n = 4 to 9 donors per genotype, 10 recipients per donor group.
Lack of osteoblastic N-cadherin does not alter numbers of functional HSCs. Analysis of the peripheral blood after competitive transplantation shows the percentage of donor cells determined by cell surface expression of CD45.2 in the total peripheral blood cell population, CD11b+ population, B220+ population, and CD3e+ population. Recipient mice were analyzed at (A) 4 weeks, (B) 8 weeks, (C) 12 weeks, and (D) 16 weeks after transplantation of donor and competitor cells. n = 4 to 9 donors per genotype, 10 recipients per donor group.
Genetic deletion of osteoblastic N-cadherin in aged mice results in decreased mineralized bone but does not change microenvironmental support for HSPCs
To determine whether the aberrant bone phenotype worsened with age and reveal osteoblastic N-cadherin effects on HSPC homeostasis, we examined 12- to 14-month-old Ob-NCadh mice and control littermates (Figure 4). This is particularly important because others have demonstrated that N-cadherin expression increases with age in both HSPC and endosteal stromal cells.10 Because a bone phenotype was detected in males predominantly, the bone phenotype was examined in aged male mice. By micro-CT analysis, we found a significant decrease in trabecular bone volume (BV/TV) in OB-NCAD mice compared with littermates (Figure 4A,D-E) without significant changes in trabecular number or thickness (data not shown), but with decreased trabecular connectivity. There were also significant decreases in cortical thickness (Figure 4C-D,F) without changes in cortical mean bone density (data not shown). No changes in serum cyclophosphamide (Figure 4G) or tartrate-resistant acid phosphatase levels (Figure 4H) were measured, suggesting no increases in bone resorption. Together, these data are consistent with a defect in osteoblastic maturation rather than changes in bone resorption, as has previously been described in other genetic models of osteoblastic N-cadherin disruption.14,22 Given the more severe bone phenotype detected in 1-year-old mice, we tested the hematopoietic phenotype in this age group and found no differences in BM cellularity (Figure 5A), peripheral blood counts (data not shown; and Figure 5G-I), spleen weights, and cell counts (Figure 5B and data not shown). Phenotypic analysis of HSPC subsets did not demonstrate a genotype differences in LSK, ST-HSCs, or LT-HSCs (Figure 5C). Competitive repopulation assays using donor WT or OB-NCadh did not uncover any differences in short- or long-term reconstitution (Figure 5D-F), again demonstrating that osteoblastic N-cadherin is not required for microenvironmental support of HSPCs. Notably, there was a significant increase in the T-cell engraftment, which was increased at 22 weeks in recipient of OB-NCAD donor cells, which could not be explained by HSC changes since myeloid and B-cell engraftment was not genotype dependent. To test HSPC function in vivo, aged WT and OB-NCadh mice were irradiated with sublethal (6.5 Gy) total body irradiation at day 0 and hematopoietic recovery was monitored. There was no genotype-dependent change in recovery in hematocrit (Figure 5G), WBC (Figure 5H), or platelets (Figure 5I). These data demonstrate that osteoblastic N-cadherin is not required for in vivo HSPC function during BM recovery from myeloablative injury.
Lack of osteoblastic N-cadherin results in decreased mineralized trabecular BV/TV and cortical thickness at 1 year of age. (A-C) Quantitative analysis of micro-CT scans depicting trabecular BV/TV (A), trabecular connectivity density (B), and cortical thickness (C). *P < .05. **P < .01. n = 3 or 4 male mice per group. (D) Representative micro-CT scans of the distal femur (top), knee joint and proximal tibia (bottom), of WT (left) and OB-NCadh (right) mice. (E) Representative micro-CT scans of trabecular bone at the metaphysis of the femur (top) or tibia (bottom) of WT (left) and OB-NCadh (right) mice. (F) Representative micro-CT scans of femur cortical bone from WT (left) and OB-NCadh (right) mice. (G-H) Quantitation of serum CTX (G) and tartrate-resistant acid phosphatase (H) levels in 1-year-old mice. Each dot represents an individual mouse; data are mean ± SEM.
Lack of osteoblastic N-cadherin results in decreased mineralized trabecular BV/TV and cortical thickness at 1 year of age. (A-C) Quantitative analysis of micro-CT scans depicting trabecular BV/TV (A), trabecular connectivity density (B), and cortical thickness (C). *P < .05. **P < .01. n = 3 or 4 male mice per group. (D) Representative micro-CT scans of the distal femur (top), knee joint and proximal tibia (bottom), of WT (left) and OB-NCadh (right) mice. (E) Representative micro-CT scans of trabecular bone at the metaphysis of the femur (top) or tibia (bottom) of WT (left) and OB-NCadh (right) mice. (F) Representative micro-CT scans of femur cortical bone from WT (left) and OB-NCadh (right) mice. (G-H) Quantitation of serum CTX (G) and tartrate-resistant acid phosphatase (H) levels in 1-year-old mice. Each dot represents an individual mouse; data are mean ± SEM.
Lack of osteoblastic N-cadherin does not alter steady-state hematopoiesis or functional HSPCs in aged mice. (A) Total marrow cellularity as determined by counting flushed marrow cells (n = 3-6 mice per group). (B) Spleen weight in grams (n = 3-6 mice per group). (C) Frequency of the LSK, MPP/ST-HSC, and LT-HSC populations as defined in Figure 2. N = 2 WT, 6 OB-NCadh 1-year-old mice. (D-F) Analysis of the peripheral blood after competitive transplantation shows the percentage of donor cells determined by cell surface expression of CD45.2 in the total peripheral blood cell population, CD11b+ population (D), B220+ population (E), and CD3e+ population (F). Recipient mice were analyzed at 4 to 22 weeks after transplantation of donor and competitor cells. *P < .05 at 22 weeks only by Bonferroni posttest analysis. No significant differences were noted by 2-way ANOVA with repeated-measures analysis using the Bonferroni posttest after transplantation of donor and competitor cells. n = 3 WT and 4 OB-NCAD donors per genotype, 13 to 20 recipients per donor group (4 or 5 recipients for each individual donor). (G-I) Hematopoietic recovery after sublethal (6.5 Gy) total body irradiation: hematocrit (G), WBCs (H), and platelets (I). No significant differences were noted between genotypes by 2-way ANOVA. n = 3 to 5 mice per genotype per time point.
Lack of osteoblastic N-cadherin does not alter steady-state hematopoiesis or functional HSPCs in aged mice. (A) Total marrow cellularity as determined by counting flushed marrow cells (n = 3-6 mice per group). (B) Spleen weight in grams (n = 3-6 mice per group). (C) Frequency of the LSK, MPP/ST-HSC, and LT-HSC populations as defined in Figure 2. N = 2 WT, 6 OB-NCadh 1-year-old mice. (D-F) Analysis of the peripheral blood after competitive transplantation shows the percentage of donor cells determined by cell surface expression of CD45.2 in the total peripheral blood cell population, CD11b+ population (D), B220+ population (E), and CD3e+ population (F). Recipient mice were analyzed at 4 to 22 weeks after transplantation of donor and competitor cells. *P < .05 at 22 weeks only by Bonferroni posttest analysis. No significant differences were noted by 2-way ANOVA with repeated-measures analysis using the Bonferroni posttest after transplantation of donor and competitor cells. n = 3 WT and 4 OB-NCAD donors per genotype, 13 to 20 recipients per donor group (4 or 5 recipients for each individual donor). (G-I) Hematopoietic recovery after sublethal (6.5 Gy) total body irradiation: hematocrit (G), WBCs (H), and platelets (I). No significant differences were noted between genotypes by 2-way ANOVA. n = 3 to 5 mice per genotype per time point.
Intensive PTH treatment increases bone turnover and trabecular bone volume
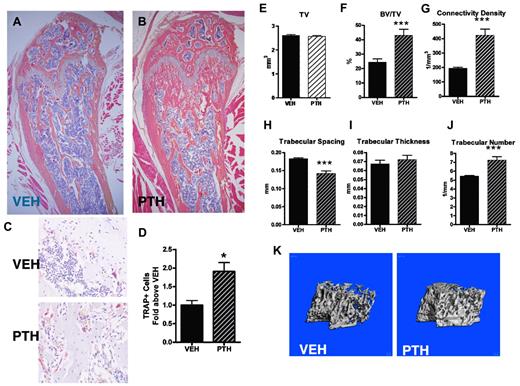
We and others have demonstrated that intermittent PTH treatment achieves HSC expansion through microenvironmental activation.1,23,24 With the goal of rapidly achieving both skeletal and hematopoietic effects, we devised an accelerated protocol of PTH treatment modifying our previous regimen.23 To activate the BM microenvironment, we treated mice in vivo with either VEH or PTH (40 μg/kg 3 times daily for 10 days). This intensive regimen was well tolerated without morbidity, mortality, or weight changes in the PTH-treated mice (data not shown). Several studies have demonstrated that intermittent treatment with PTH is anabolic to bone.25 To confirm that our intensive PTH treatment is anabolic to bone, we performed micro-CT analysis of both femora and tibiae of VEH- and PTH-treated mice. In this model of PTH treatment, trabecular architecture was altered with increased trabecular structures visualized by histologic analysis (Figure 6A-B). Tartrate-resistant acid phosphatase (TRAP) staining of paraffin sections from VEH- or PTH-treated mice also revealed a significant increase in TRAP+ osteoclastic cells after PTH treatment (Figure 6C-D). Analysis of micro-CT scanning demonstrated significant increases in trabecular bone at the metaphysis of the distal femur, including increased BV/TV, connectivity density, number of trabeculae, and a corresponding decrease in trabecular spacing, whereas trabecular thickness remained unchanged (Figure 6E-J). Three-dimensional reconstructions of micro-CT scans from the distal femora in the trabecular region also demonstrated increased trabecular structures (Figure 6K). Analysis of tibiae revealed a similar phenotype; and interestingly, there were no differences in cortical thickness or density in PTH-treated mice, as measured by micro-CT analysis (supplemental Figure 1). These data suggest increased bone turnover and demonstrate increased mineralized trabecular bone and no significant cortical changes as a consequence of the intensive PTH treatment.
PTH treatment (3 times a day) increases trabecular bone volume and osteoclastic cells. (A-B) Representative images of H&E-stained paraffin sections from the distal femur of (A) VEH-treated and (B) PTH-treated mice. Images were obtained using a 4× objective. (C) Representative images of TRAP-stained paraffin sections from VEH-treated (top panel) and PTH-treated (bottom panel) mice. Sections were counterstained with hematoxylin. Images were obtained using a 20× objective. (D) Quantification of TRAP+ cells from paraffin sections represented by panel C. (E-J) Micro-CT analysis of trabecular bone from the femur of VEH- and PTH-treated mice, including (E) total volume, (F) bone volume/total volume, (G) connectivity density, (H) trabecular spacing, (I) trabecular thickness, and (J) trabecular number. (K) Representative micro-CT images of trabecular bone from the femur of VEH- or PTH-treated mice. *P < .05. **P < .01. ***P < .001. n = 5 or 6 per treatment group.
PTH treatment (3 times a day) increases trabecular bone volume and osteoclastic cells. (A-B) Representative images of H&E-stained paraffin sections from the distal femur of (A) VEH-treated and (B) PTH-treated mice. Images were obtained using a 4× objective. (C) Representative images of TRAP-stained paraffin sections from VEH-treated (top panel) and PTH-treated (bottom panel) mice. Sections were counterstained with hematoxylin. Images were obtained using a 20× objective. (D) Quantification of TRAP+ cells from paraffin sections represented by panel C. (E-J) Micro-CT analysis of trabecular bone from the femur of VEH- and PTH-treated mice, including (E) total volume, (F) bone volume/total volume, (G) connectivity density, (H) trabecular spacing, (I) trabecular thickness, and (J) trabecular number. (K) Representative micro-CT images of trabecular bone from the femur of VEH- or PTH-treated mice. *P < .05. **P < .01. ***P < .001. n = 5 or 6 per treatment group.
Intensive PTH treatment expands HSPCs in the marrow
Previous studies have demonstrated that intermittent PTH treatment results in increased HSPCs.1,23,24 Our current intensive PTH treatment regimen recapitulated these findings with significant increases in phenotypic populations of Flt3+CD48−CD150− LSKs enriched for MPP/ST-HSCs (Figure 7A) as well as Flt3−CD48−CD150+ LSKs enriched for LT-HSCs (Figure 7B). In addition, a separate phenotypic analysis using Flt3 and Thy1.1 cell surface expression was used to separate MPPs and ST-HSCs (Figure 7C-E). PTH-treated mice demonstrated increased levels of Flt3+Thy1.1low cells enriched for MPPs as well as Flt3+Thy1.1hi cells enriched for ST-HSCs (Figure 7F-G). Functional increases in short-term and long-term repopulation were also demonstrated in mice treated with PTH. Primary competitive transplantation revealed increased repopulation in recipients of marrow cells from PTH-treated donors through 12 weeks (Figure 7H). Secondary transplants were also performed and revealed greatly increased engraftment from the original PTH-treated donors through 16 weeks (Figure 7I). These data suggest that our short, intensive PTH treatment regimen is sufficient to expand HSPCs.
PTH treatment (3 times a day) increases LT- and ST-HSCs both phenotypically and functionally. (A) Frequency of MPP/ST-HSCs from VEH- or PTH-treated mice based on cell surface expression of CD150 and CD48, as shown in Figure 2. (B) Phenotypic quantification of LT-HSCs based on surface expression of CD150 and CD48, as shown in Figure 2. (C-E) Representative flow cytometry plots depicting the isolation of MPP, ST-HSCs, and LT-HSCs. (F) Frequency of the MPP population of cells as defined by panel E. (G) Frequency of the ST-HSC population of cells as defined by panel E. (H) Analysis of the peripheral blood after primary competitive transplantation shows the percentage of donor cells determined by cell surface expression of CD45.1 in the CD11b+, B220+, and CD3e+ population of cells. Recipient mice were analyzed at 3 weeks (top panel), 6 weeks (middle panel), and 12 weeks (bottom panel) after transplantation of donor and competitor cells. (I) Analysis of the peripheral blood from secondary transplantation recipient mice was performed at 6 weeks (top panel), 12 weeks (middle panel), and 16 weeks (bottom panel) after transplantation. *P < .05. **P < .01. ***P < .001. n = 6 per treatment group, 3 donors per treatment group, 10 to 20 recipients per treatment group.
PTH treatment (3 times a day) increases LT- and ST-HSCs both phenotypically and functionally. (A) Frequency of MPP/ST-HSCs from VEH- or PTH-treated mice based on cell surface expression of CD150 and CD48, as shown in Figure 2. (B) Phenotypic quantification of LT-HSCs based on surface expression of CD150 and CD48, as shown in Figure 2. (C-E) Representative flow cytometry plots depicting the isolation of MPP, ST-HSCs, and LT-HSCs. (F) Frequency of the MPP population of cells as defined by panel E. (G) Frequency of the ST-HSC population of cells as defined by panel E. (H) Analysis of the peripheral blood after primary competitive transplantation shows the percentage of donor cells determined by cell surface expression of CD45.1 in the CD11b+, B220+, and CD3e+ population of cells. Recipient mice were analyzed at 3 weeks (top panel), 6 weeks (middle panel), and 12 weeks (bottom panel) after transplantation of donor and competitor cells. (I) Analysis of the peripheral blood from secondary transplantation recipient mice was performed at 6 weeks (top panel), 12 weeks (middle panel), and 16 weeks (bottom panel) after transplantation. *P < .05. **P < .01. ***P < .001. n = 6 per treatment group, 3 donors per treatment group, 10 to 20 recipients per treatment group.
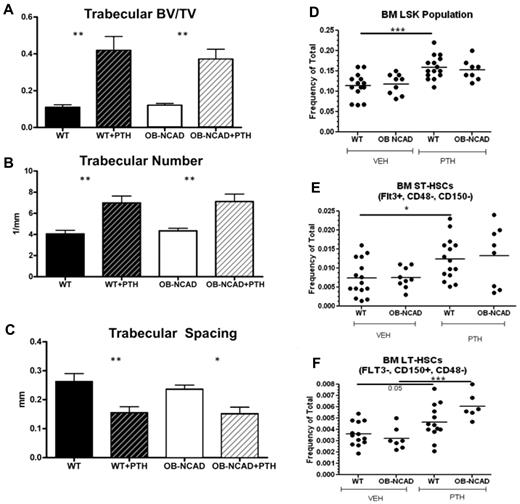
Osteoblastic N-cadherin is not required for PTH-dependent increase in trabecular bone or expansion of HSPCs
Although osteoblastic N-cadherin is not required for HSC maintenance or steady-state hematopoiesis, it may be necessary for the expansion of the HSPC population in the marrow. To test this, we treated OB-NCadh mice and WT littermate controls with VEH or PTH using our short, intensive regimen. As previously noted, trabecular changes were not found in VEH-treated 2- to 3-month-old OB-NCadh mice (Figure 8A-C). Loss of osteoblastic N-cadherin did not affect the ability of PTH to increase trabecular structures (Figure 8A-C). In addition, phenotypic analysis of HSPC populations in the marrow revealed that loss of osteoblastic N-cadherin did not affect the ability of PTH to expand the population of LT- or ST-HSCs (Figure 8D-E). These data suggest that osteoblastic N-cadherin is not required for PTH-dependent expansion of HSPCs in the marrow. Unexpectedly, larger variability in percent donor engraftment was noted with PTH treatment in the WT, CD45.2 mice compared with the results obtained in the nongenetically altered CD45.1-treated mice (Figure 7), which appeared to have a milder effect. When reconstitution was quantified based on the proportion of recipients demonstrating more than or equal to 1% donor engraftment, again there was no difference between WT and OB-NCadh that were VEH-treated (15 of 16 vs 13 of 19, P > .05). However, in OB-NCadh mice, there was a significant increase in the proportion of recipients demonstrating donor engraftment with PTH donor treatment (13 of 19 vs 20 of 20, VEH vs PTH treated OB-NCadh mice, P = .0083). These data demonstrate that osteoblastic N-cadherin is not required for PTH-dependent HSPC expansion.
Lack of osteoblastic N-cadherin does not inhibit the ability of PTH treatment to increase trabecular bone or HSC subsets. (A-C) Micro-CT analysis of trabecular bone from the femur of VEH- and PTH-treated WT and OB-NCadh mice: (A) trabecular bone volume/total volume, (B) trabecular number, and (C) trabecular spacing. (D-F) Frequency of LSK (D), ST-HSCs (E), and LT-HSCs (F), as defined in Figure 2 in the BM of WT and OB-NCadh mice treated with VEH or PTH. *P < .05. **P < .01. ***P < .001. Each dot indicates an individual mouse.
Lack of osteoblastic N-cadherin does not inhibit the ability of PTH treatment to increase trabecular bone or HSC subsets. (A-C) Micro-CT analysis of trabecular bone from the femur of VEH- and PTH-treated WT and OB-NCadh mice: (A) trabecular bone volume/total volume, (B) trabecular number, and (C) trabecular spacing. (D-F) Frequency of LSK (D), ST-HSCs (E), and LT-HSCs (F), as defined in Figure 2 in the BM of WT and OB-NCadh mice treated with VEH or PTH. *P < .05. **P < .01. ***P < .001. Each dot indicates an individual mouse.
Discussion
Much evidence, including our own, implicates cells of the osteoblastic lineage as a targetable HSC niche component.3,6,23,26 The signals mediating these regulatory interactions are beginning to be elucidated. Much controversy remains on the role of N-cadherin in HSC-microenvironmental interactions; yet despite attempts at resolving this debate,13 unexplored areas remain. In this manuscript, we address 2 distinct questions previously unresolved: (1) whether osteoblastic deletion of N-cadherin alters the marrow microenvironment, and (2) whether such genetic disruption changes the hormonal action of PTH on the marrow microarchitecture and/or on resident HSPCs. Here, we demonstrate that targeted deletion of Cdh2 in maturing osteoblastic cells progressively alters the bone microenvironment without changes in the hematopoietic system. Moreover, when the HSC niche was stimulated by using an accelerated PTH regimen that achieved rapid skeletal changes, as well as expansion of both ST- and LT-HSCs, lack of osteoblastic N-cadherin did not block either of these actions of PTH.
Previous data have demonstrated that inducible global lack of N-cadherin is not required for hematopoiesis.11 For these experiments, the authors used the well-established Mx1-Cre transgenic line, which is inducible through activation by poly(I:C)/IFN-α,27 and N-cadherinfl/− mice.18 However, microenvironmental deficiency of N-cadherin was not assessed. Moreover, recent data have shown that poly(I:C)/IFN-α can activate dormant HSCs in vivo,28 a phenomenon that may have obscured effects of Cdh2 deletion on HSC function. We directly targeted the osteoblastic lineage by using Col1-Cre transgenic mice17 because this specific promoter affects osteoblastic cells that initiated PTH action on the HSC niche,20,23 and demonstrate lack of osteoblastic N-Cad protein in vivo. We chose not to use N-cadherin+/− in this mating because global hemizygosity results in osteoblastic defects,16 which may have affected osteoblastic support of HSCs and/or PTH action.
Lack of osteoblastic N-cadherin did not change skeletal morphology at birth or bone volume and microarchitecture in male juvenile (3 months old) and female mice. Changes in trabecular microarchitecture were detected in older male mice, initially with increased trabecular volume and number, and later in aged mice, with decreased mineralized trabecular bone and cortical thickness. These data support the inhibitory role of N-cadherin in terminal osteoblastic maturation, which has been suggested by analysis of Col1-Cre Ncadherinfl/− in vitro.14 Our results also agree with an inhibitory role of N-cadherin on osteoblastic maturation through negative regulation of Wnt/β-catenin demonstrated in vivo in mice overexpressing N-cadherin in osteoblastic cells.22 Cadherins participate in complexes with catenins, which are known to modulate Wnt signaling,14,29,30 which has been demonstrated to have well-documented effects on osteoblastic maturation.31 Further evaluation is required in our model to determine whether our phenotype indeed depends on increased osteoblastic Wnt/β catenin signaling. Because other cadherins, principally cadherin11 but also P-cadherin (albeit at lower levels),32 are also expressed in osteoblastic cells, we cannot exclude a compensatory effect in response to lack of N-cadherin.14 However, because the main effects of the other principal osteoblastic cadherin, cadherin11, appear to favor osteogenesis compared with adipogenesis and have only mild effects on late osteoblasts,33 significant effects on maturing osteoblastic cells would not be expected. The presence of trabecular changes in males and not females lacking osteoblastic N-cadherin was unexpected. This was a novel finding compared with the previous report by one of the authors (R.C.)14 demonstrating smaller long bones but no trabecular changes when Cdh2-/fl;Col1Cre were analyzed. We attribute this difference to the presence of a germline null allele, with possible systemic effects as well as mild osteoblastic defects.16 Moreover, the increased trabecular volume and number we detected in the present study became evident only when male and female mice were analyzed independently. In addition, this phenotype differs from that seen in a previous report using a dominant negative N-cadherin.34 We attribute this difference to the use of a different promoter driving the genetic alterations. Further, dominant negative N-cadherin did not specifically inhibit the signaling of N-cadherin and exerted its effects on other cadherin family member proteins as well. Our report adds to numerous data35,36 demonstrating sex-specific changes in the skeleton as a result of identical genetic manipulations, which would otherwise not have been anticipated to alter hormonal status or display sex dimorphism.
Our data strongly suggested that N-cadherin presence in maturing osteoblastic cells is not required for HSC maintenance. These results are consistent with a companion paper in which Greenbaum et al delete Cdh2 in a more primitive osteoblastic population using the Osx-Cre, and convincingly demonstrate no hematopoietic effects,37 and with the results from Kiel et al.11 Our data do not discount the possibility that N-cadherin expression on other cell types implicated in the HSC niche, such as endothelial cells, and Nestin+ mesenchymal cells, could be important in the regulation of HSCs. Further it is well established that osteolineage cells targeted in this report are essential components of the HSC niche, as their depletion under a similar promoter results in hematopoietic defects.26 Together, our data establish definitively that lack of N-cadherin in cells of the osteoblastic lineage has no effect on immature and mature hematopoiesis.
In previous work, mice injected daily with PTH(1-34) for a month had a significant increase in functional HSCs without other changes in hematopoiesis.23 We devised an accelerated protocol to rapidly achieve a bone anabolic effect and here demonstrate strong PTH anabolic action as well as the previously demonstrated increase in HSCs with long-term engraftment. The action of PTH on long-term HSCs using this novel accelerated method is consistent with both our previous reports using in vivo PTH treatment.1,23 However, in that initial evaluation, the effect of osteoblastic activation on phenotypic stem cell subsets was not directly tested. The effect of PTH and osteoblastic activation/expansion on individual HSC subsets is of great importance because it could provide a cellular mechanism for HSC expansion if PTH increases LT-HSC abundance by altering the proportion HSC subsets. In addition, differential PTH effects on HSC subsets may reveal independent niches capable of regulation of specific HSC subsets. The current data demonstrate the effects of PTH on the LSK subset when it is further subdivided to phenotypically identify the long-term and short-term populations using the SLAM receptors CD48/CD1507 and Flt-3.38 PTH increased phenotypic LT- and ST-HSPCs, and these increases were confirmed by improvement in both short-term and long-term engraftment induced by PTH treatment in the donor mice. Unexpectedly, there was a more robust PTH effect in CD45.1 compared with CD45.2 mice, further supporting previously reported fundamental differences in HSPCs in these congenic strains. The current study therefore extends our previous studies and establishes PTH-dependent increases on both short-term and long-term phenotypic HSPCs.
When this accelerated PTH regimen was used in mice lacking osteoblastic N-cadherin, we observed no measurable inhibition in either the bone anabolic effect of PTH or in the effect of PTH on phenotypic and functional HSPCs. Therefore, our data demonstrate that the presence of N-cadherin in maturing osteoblastic cells is not required for these effects of PTH on the marrow and its microarchitecture. Because activation of the PTH in maturing osteoblastic cells was sufficient to initiate the PTH-dependent marrow effects, N-cadherin is also not required as a signal that initiates PTH anabolic bone action or its effects on the HSC niche. However, given recent data on the role of early osteoprogenitors39 and mesenchymal stem cells24 on HSC support, it is possible that N-cadherin on mesenchymal stem cells may be required for some of the actions of PTH mediated by other cells in the HSCs niche beyond osteoblastic cells.
In conclusion, we demonstrate that osteoblastic N-cadherin is important for the marrow microarchitecture but not required for normal hematopoiesis, PTH bone anabolic action, or activation of the HSC niche by PTH. Our data, in conjunction with that of Greenbaum et al,37 firmly establish that osteoblastic N-cadherin is not required for normal hematopoiesis. However, we cannot discount the possibility that other cadherin family molecules are compensating for the conditional loss of N-cadherin. In addition, these studies demonstrate that, in at least some instances of modulation of the niche (response to myeloablative radiation injury, PTH-dependent HSC expansion, and G-CSF–induced mobilization), osteoblastic N-cadherin is dispensable. HSCs are currently the only stem cells routinely used for therapeutic purposes where clinical experience has shown that HSC number is an important limiting factor in treatment success. Strategies to expand HSCs through manipulation of niche are of great clinical appeal; therefore, further evaluation of the HSC microenvironment, particularly through the use of specific genetic models, should continue to refine our definition of HSC niche components.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr G. Karsenty (Columbia University, New York, NY) and Dr G. Radice (Thomas Jefferson University, Philadelphia, PA) for providing genetically altered mice, Dr H. Awad (University of Rochester School of Medicine) for optimizing micro-CT analysis, and Dr T. Love (University of Rochester School of Medicine) for providing statistical support.
This work was supported by the National Institutes of Health, National Institute of Diabetes, Digestive and Kidney Diseases (R01 DK 076876, L.M.C.).
National Institutes of Health
Authorship
Contribution: O.B., B.J.F., J.M.W., and R.L.P. performed experiments; B.J.F., L.M.C., O.B., and J.M.W. analyzed experiments; B.J.F. and L.M.C. wrote the manuscript; and B.J.F., L.M.C., O.B., J.M.W., and R.C. discussed data and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Laura M. Calvi, Endocrine Division, Department of Medicine, University of Rochester School of Medicine, 601 Elmwood Ave, Box 693, Rochester, NY 14642; e-mail: laura_calvi@urmc.rochester.edu.
References
Author notes
O.B. and B.J.F. contributed equally to this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal