In this issue of Blood, Safeukui et al have come to grips with an important issue in red blood cell (RBC) biology.1 Their study deals not only with the mechanism of RBC removal in diseases like hereditary spherocytosis (HS) and autoimmune hemolytic anemia, but also with RBC senescence.2
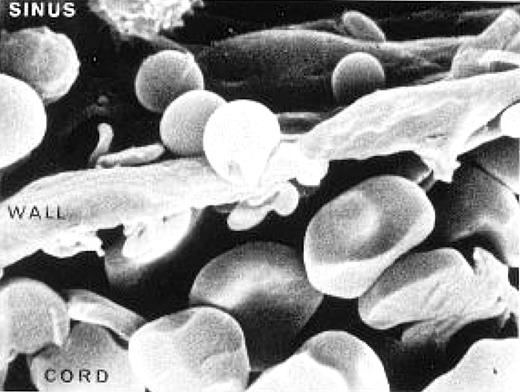
To understand their contribution, first consider the evolutionary beauty of our bi-concave discocytic RBC. A sphere with a volume of approximately 90 femtoliters (the volume/mean corpuscular volume [MCV] of a normal RBC) would have a surface area of 95 square microns. By actual measurement the surface area of an RBC is 140 square microns, giving an excess surface area of 45 square microns—this is the surface area volume (S/V) ratio beloved by red cell hematologists who somehow missed the future belonging to Flt3 and NPM1. It is this excess surface area that allows the RBC with a diameter of 7 to 8 microns to elliptically deform to a length 5 times its width and tank tread its way down 3- to 5-micron-diameter capillaries and through 3-micron slits in the sinus-endothelial wall of the reticulo-endothelial organs (see figure). A tennis ball cannot elliptically deform, but a biconcave discocyte with excess surface area can. But it is not only S/V considerations that determine RBC deformability. There are two other important determinants: the internal viscosity of the RBC reflected in the mean corpuscular hemoglobulin concentration (MCHC), that is, the concentration of densely packed hemoglobin molecules normally approximately 35 g/dL (consider that we worry about serum viscosity in our myeloma patients when the globulin reaches levels of 6 gm/dL); and the intrinsic properties of the proteins and lipids that make up the RBC membrane.
Rat spleen showing a red blood cell leaving the cord and deforming to pass through a slit in the wall of the sinus. Slide provided by Dr Mohandas Narla. Reproduced with permission from: Mohandas Narla. Red cell membrane dynamics and organization. In: UpToDate, Basow, DS (Ed), UpToDate, Waltham, MA 2012. Copyright © 2012 UpToDate, Inc. For more information visit www.uptodate.com.
Rat spleen showing a red blood cell leaving the cord and deforming to pass through a slit in the wall of the sinus. Slide provided by Dr Mohandas Narla. Reproduced with permission from: Mohandas Narla. Red cell membrane dynamics and organization. In: UpToDate, Basow, DS (Ed), UpToDate, Waltham, MA 2012. Copyright © 2012 UpToDate, Inc. For more information visit www.uptodate.com.
We have long believed that this critical S/V ratio was monitored by the reticulo-endothelial system, particularly in the complex vasculature of the spleen, where the RBCs are first discharged into the cords of Billroth and then have to elliptically deform to pass into the sinuses through slits in the sinus wall (see figure). All this happens while adjacent macrophages are looking on and performing quality control. This belief in the role of the spleen has been fostered by the generally successful clinical use of splenectomy in HS and somewhat less successful use in autoimmune hemolytic anemia. A recurrent biologic question has always been, “What does the spleen see?” Is it RBC surface charge, or membrane-attached immunglobulins or complement, or can the spleen actually sense deformability and spheroidicity?
Safeukei and colleagues have cleverly designed a method for perfusing recently removed human spleens with tagged RBCs. Their idea was to test a hypothesis concerning the mechanism of splenic sequestration or splenic entrapment of RBCs that have been altered to reduce their deformability. They used a trick well known to red cell hematologists; that is, incubating RBCs with lysophosphatidylcholine (LPC). The LPC intercalates specifically into the outer half of the RBC membrane phospholipid bilayer, causing it to undergo outward expansion, producing first echinocytosis and then after the spicules fall off, spherocytosis. The amount of LPC and the incubation time can be altered to produce varying degrees of spherocytosis. In these experiments the MCHC was not altered so that increases in internal viscosity could not contribute to the reduction in deformability. Further, there were no alterations in the RBC membrane proteins. Therefore, the reduced deformability3 of these LPC-treated RBCs was directly related only to S/V considerations or spheroidicity. The authors showed that graded RBC surface area loss lead to parallel increases in splenic entrapment. Therefore, the spleen can see loss of S/V and spheroidicity.
Interestingly, Safeukei et al then noted a heterogeneity in removal of apparently equivalently spherocytic RBCs, leading them postulate that deformation may cause a leak of intracellular ions that causes a loss in RBC volume, restoring the S/V. In fact, there are mechanically activated ion channels in RBCs,4 so the hypothesis is reasonable.
These experiments were designed with the idea that the alterations in RBCs induced by LPC might mimic those seen in HS. But as Safeukei and colleagues point out, the mimicry is not exact because HS RBCs frequently have an increased MCHC, the cause of which is not clear. In addition, we know from use of the eosin-maleimide diagnostic test that there are further mysteries in the patho-biology of HS.5 The eosin-maleimide test seems to have increased diagnostic accuracy because it not only detects S/V reduction but also the re-duced access of eosin-maleimide to CD 47, band 3, and Rh protein. Why this should occur in HS is not clear.
We look forward to see use of this experimental design in studying splenic RBC removal in autoimmune hemolytic anemia.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■