In this issue of Blood, Bromberg et al1 and Greenbaum et al2 delete the gene encoding the cell adhesion molecule N-cadherin in osteoblastic cells forming the hematopoietic stem cell (HSC) niche to demonstrate that N-cadherin has no role in regulating hematopoiesis.
Ex vivo expansion of transplantable HSCs has been thwarted by the rapid differentiation of HSCs when cultured with purified recombinant cytokines. This is because key signals from the bone marrow niches that promote self-renewal instead of differentiation are missing. Identification of such niche signals would facilitate ex vivo HSC expansion, which would reduce the number of patients who cannot proceed to transplantation because of too low hematopoietic stem and progenitor cell numbers in the graft. Intense efforts have been devoted to identify the cellular and molecular components of HSC niches in the bone marrow. Although HSC niches were described more than 3 decades ago, their identification and molecular characterization has proven technically challenging due to the rarity of HSCs in the marrow (∼ 1 HSC per 20 000 marrow cells), and the paucity of unique identifying markers. With the advent of more sophisticated microscopy and imaging techniques, phenotypic HSCs were found to be enriched in the endosteal region of the marrow, within 2 cell diameters from osteoblasts3,4 and/or adjacent to the abluminal side of sinusoidal endothelial cells5 in mice. This has led to the concepts of endosteal (or osteoblastic) and perivascular niches supporting HSCs. Whether osteoblastic and perivascular niches are functionally distinct and which molecular components regulate HSC quiescence or self-renewal are under intense investigation, made more difficult as these locations often overlap in the richly vascularized endosteum.6
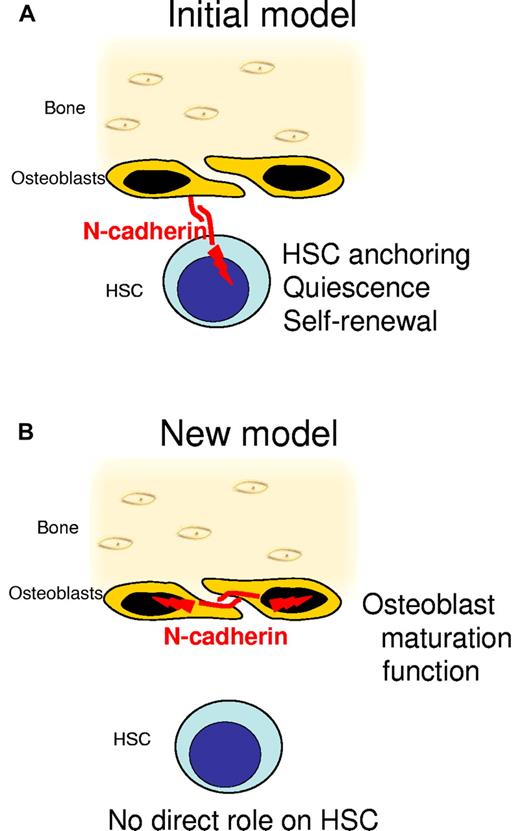
In one of the first 2 Nature papers identifying the osteoblastic niche, quiescent HSCs were found in contact with unusual spindle-shaped osteoblasts.3 Confocal microscopy with anti–N-cadherin antibodies revealed that both spindle-shaped osteoblasts and quiescent HSCs express N-cadherin.3 As cadherins promote cell-cell adhesion by binding to cadherins expressed by adjacent cells, it was tantalizing to speculate the N-cadherin molecules expressed on osteoblasts and HSCs anchor HSCs within their niche by homotypic adhesion and promote HSC quiescence (see figure). In support of this, enforced expression of wild-type N-cadherin into HSCs increased the lodgment of transplanted HSCs in the endosteal region and their self-renewal in vivo,7 whereas enforced expression of dominant negative mutant7 or knockdown of the N-cadherin gene (Cdh2) in HSCs8 caused the opposite with reduced lodgment at the endosteum, accelerated proliferation, and more rapid exhaustion of transplanted HSCs. This model was, however, seriously challenged by the findings that (1) N-cadherin mRNA is undetectable in sorted HSCs,9 (2) N-cadherin antibodies used in immunohistology may have specificity issues,9 and (3) conditional deletion of the N-cadherin gene in hematopoietic cells had no effect on their homing, engraftment, and long-term reconstitution capability.10 However, as N-cadherin can also bind to other structurally related cadherins, the possibility that N-cadherin expressed by osteoblasts could regulate HSCs via other nonidentified cadherins expressed by HSCs remained.11
N-cadherin regulates osteoblasts but not HSCs. (A) The initial observation of the osteoblastic niche suggested a model in which N-cadherin was expressed by both spindle-shaped osteoblasts and hematopoietic stem cells (HSCs). Homotypic adhesion between N-cadherin expressed by osteoblasts and N-cadherin expressed by HSCs enables HSC anchoring in the niches and signals quiescence and self-renewal to HSCs. (B) In the new model, N-cadherin is only expressed by osteoblasts, not by HSCs. Deletion of N-cadherin in osteoblasts alters osteoblast function and maturation but has no effect on HSC behavior.
N-cadherin regulates osteoblasts but not HSCs. (A) The initial observation of the osteoblastic niche suggested a model in which N-cadherin was expressed by both spindle-shaped osteoblasts and hematopoietic stem cells (HSCs). Homotypic adhesion between N-cadherin expressed by osteoblasts and N-cadherin expressed by HSCs enables HSC anchoring in the niches and signals quiescence and self-renewal to HSCs. (B) In the new model, N-cadherin is only expressed by osteoblasts, not by HSCs. Deletion of N-cadherin in osteoblasts alters osteoblast function and maturation but has no effect on HSC behavior.
To resolve the N-cadherin controversy,11 Bromberg et al1 and Greenbaum et al2 undertook to conditionally delete the N-cadherin gene in the osteoblastic lineage to determine the effect of osteoblastic N-cadherin on hematopoiesis in vivo. While the former group deleted the N-cadherin gene in maturing osteoblasts expressing collagen I, the latter group deleted N-cadherin much earlier in the osteoblastic lineage, in primitive osteoprogenitors at early phases of osteoblastic commitment using the promoter of the osterix gene, which is necessary to commit mesenchymal progenitor cells to the osteoblastic lineage. Remarkably in both studies, deletion of the N-cadherin gene in osteoprogenitors or more mature osteoblasts had no effect on HSC number, cycling, or differentiation potential in steady-state; no effect on HSC retention within their niche in steady-state or after mobilization with G-CSF; no effect on hematopoietic recovery after cytotoxic stress; and no effect on HSC engraftment or self-renewal after transplantation. Furthermore, neither bone formation stimulation nor the associated expansion of the HSC pool that occurs in response to para-thyroid hormone treatment was altered by deletion of the N-cadherin gene in maturing osteoblasts. This is in contrast with the observation that in steady-state, deletion of the N-cadherin gene in either osteoprogenitors or maturing osteoblasts alters osteoblast function. Deletion of N-cadherin in osteoprogenitors reduced trabecular bone density whereas deletion of N-cadherin in osteoblasts increased trabecular bone density before decreasing it in older mice. Therefore, osteoblastic N-cadherin regulates osteoblast maturation and function but has no effect on the hematopoietic system in vivo. These findings are congruent with the previously reported lack of effect of N-cadherin deletion in the hematopoietic lineage.10
While closing the N-cadherin debate, the 2 papers in this issue of Blood fuel another controversy in respect to the role of osteoblastic niches versus perivascular HSC niches. Indeed, both papers give another example of genetically induced alteration of bone formation and osteoblast function without an effect on HSCs. Is it because N-cadherin is not important in the osteoblastic niche or rather that HSCs are also regulated by endothelial,12 mesenchymal,12,13 and glial cells14 in the perivascular niche?6 And if these perivascular niches are more important, what would the effect of N-cadherin gene deletion in perivascular cells be? The long quest of identifying the niche components that critically regulate HSC quiescence and self-renewal in the bone marrow is far from over, but at least we now know that N-cadherin expressed by osteoblastic cells is not necessary in vivo.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal