Abstract
The clinical goal of allogeneic hematopoietic stem cell transplantation (allo-HSCT) is to minimize GVHD while maintaining GvL. Here, we show that interferon γ receptor-deficient (IFNγR−/−) allogeneic Tconv, which possess normal alloreactivity and cytotoxicity, induce significantly less GVHD than wild-type (WT) Tconv. This effect is mediated by altered trafficking of IFNγR−/− Tconv to GVHD target organs, especially the gastrointestinal (GI) tract. We show that the chemokine receptor CXCR3 is induced via IFNγR-mediated signaling and partially contributes to the trafficking of WT Tconv to GVHD target organs. Indeed, CXCR3−/− Tconv recapitulate the reduced GVHD potential of IFNγR−/− Tconv in a minor-mismatched GVHD model. Most importantly, IFNγR−/− (and CXCR3−/−) Tconv mediate a robust and beneficial GvL effect. In addition, we show that IFNγR−/− regulatory T cells (Tregs) are fully suppressive in vitro although defective in suppressor function in vivo and that WT Tregs suppress GVHD in vivo only when allogeneic Tconv produce interferon γ (IFNγ), suggesting that the IFNγR signaling pathway is the major mechanism for both Tregs and Tconv to migrate to GVHD target organs. Finally, pharmacologic inhibition of IFNγR signaling with inhibitors of JAK1/JAK2, which are mediators of IFNγR signaling, results in the decreased expression of CXCR3 and reduced GVHD and improved survival after allo-HSCT and this effect is mediated by altered trafficking of Tconv to GVHD target organs.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only curative treatment for patients with relapsed/refractory leukemia, and marrow failure states such as myelodysplasia and aplastic anemia. However, the infusion of allogeneic donor T cells (conventional T cells or “Tconv”) for allo-HSCT results in 2 distinct biologic effects: graft-versus-host disease (GVHD), which may be mild, moderate, or life-threatening1,2 ; and a beneficial graft-versus-leukemia (GvL) effect, which results in enhanced leukemia cell clearance.3,4 Thus, the clinical goal in allo-HSCT is to prevent GVHD while maintaining the beneficial GvL effect. Recent studies have suggested that this might be achieved by infusing regulatory T cells (Tregs), which in some preclinical models suppress GVHD-causing alloreactive Tconv but have only limited effects on GvL-promoting alloreactive Tconv.5-8 Unfortunately, Tregs exist in low frequency in the peripheral blood, are difficult to purify and expand, and after expansion are difficult to isolate because of the lack of cell-surface markers, all of which prevent their routine use in the clinic. Thus, alternative therapeutic approaches that do not require Tregs are needed.
Interferon γ (IFNγ) is a well-known proinflammatory cytokine. Serum levels of IFNγ after allo-HSCT have been correlated with the severity of GVHD and the treatment of murine allo-HSCT recipients with blocking antibodies to IFNγ mitigates GVHD.9-12 In addition, IFNγ facilitates T cell–mediated GvL.11 In contrast, several reports suggest that IFNγ−/− T cells induce more severe GVHD, especially in the lung, than WT T cells when infused into WT MHC-mismatched recipients that are lethally irradiated,10-14 suggesting that IFNγ may also have anti-inflammatory properties. Possible mechanisms underlying this anti-inflammatory effect of IFNγ on lung GVHD have been proposed by several groups.14-16 First, donor T cell–derived IFNγ prevents allogeneic donor T-cell trafficking and expansion in the lung by inducing PDL1 expression on host lung tissue.14,15,17 Second, donor T cell–derived IFNγ induces indoleamine 2,3-dioxygenase (IDO) expression in donor bone marrow-derived dendritic cells, which in turn suppress GVHD.16 All of these observations suggest that GVHD and GvL can be regulated by modifying the IFNγ-IFNγR signaling pathway.
In this report, we explore the role of the IFNγ-IFNγR signaling pathway in T-cell trafficking and GVHD. We show that the IFNγ-IFNγR signaling pathway mediates trafficking of both conventional T cells (Tconv) and regulatory T cells (Tregs) to GVHD target organs and sites of inflammation. Our results may further explain the pleiotropic effects of IFNγ described in the previous paragraph. We have also explored the mechanism by which the IFNγ-IFNγR signaling pathway mediates T-cell trafficking and GVHD. We show that signaling through IFNγR mediates increased surface expression of CXCR3, a key chemokine receptor involved in T-cell trafficking to sites of inflammation. Of particular interest is that genetic deletion of either IFNγR or its downstream target CXCR3 in donor T cells results in reduction of GVHD and altered T-cell trafficking to the spleen and away from the GI tract while maintaining robust engraftment and GvL or graft-versus-tumor (GvT) effects in vivo. Because signaling through the IFNγR is mediated by JAK1/JAK2 and STAT1, we hypothesized that pharmacologic inhibition of JAK1/JAK2 would phenocopy the effects we observed in IFNγR−/− donor T cells. We demonstrate this using commercially available and recently FDA-approved JAK1/JAK2 inhibitors providing the foundation for future clinical trials using these reagents as prophylaxis and treatment of GVHD in humans.
Methods
Mice
All mice, except IFNγ-deficient (−/−) and IFNγR−/− (Ifngr1−/−),18 which were provided by Herbert Virgin, Washington University School of Medicine, were obtained from The Jackson Laboratory. Animal care and euthanasia protocols were approved by the Washington University School of Medicine Animal Studies Committee.
Cell culture
Mouse pan T cells (CD4+ and CD8+ T cells; Tconv) were isolated from mouse spleens using Miltenyi microbeads and an AutoMACS (Miltenyi Biotech).19 The isolated Tconv were activated for 3 days in the presence of anti-CD3/CD28 antibody-coated beads (bead:cell = 1:1; Invitrogen) and Xcyte media20 with no IL-2. Supernatants were collected and cytokines were analyzed (Rodent Map v2.0, Rules Based Medicine).
Regulatory T cells
CD4+CD25+ cells were isolated using AutoMACS. Purified WT Tregs5-8 and IFNγR−/− Tregs were 88.6% ± 9.5 and 90.09% ± 5.5 CD4+CD25+, respectively, of which 84.8% ± 5.3 and 87.3% ± 7.7 were FOXP3+, respectively.
Flow cytometric analysis
The antibodies used for flow cytometric analyses are as follows. For human T cells: CD4, CD8, and CD183 (clone: 1C6/CXCR3; BD Pharmingen), for mouse T cells: CD4, CD8, CD119 (IFNGR1, clone: GR20), CXCR4 (clone: 2B11/CXCR4), CXCR5 (clone: 2G8; BD Pharmingen), CD183 (clone: CXCR3-173), GZMB (clone: 16G6; eBioscience), IL-21R (clone: 4A9), CXCR2 (clone: TG11/CXCR2), CCR3 (clone: TG14/CCR3), CCR4 (clone: 2G12), CCR5 (clone: HM-CCR5), CCR6 (clone: 29-2L17), CCR7 (clone: 4B12), CCR9 (clone: 9B1; BioLegend), and for allo-HSCT: H-2Kb, CD3, CD4, CD8, B220, and CD45.2 (BD Pharmingen). All cells were analyzed on a FACScan cytometer (BD Bioscience).
Mixed lymphocyte reaction
For CFSE-based mixed lymphocyte reaction (MLR), Tconv (B6, CD45.1+) were labeled with carboxyfluorescein diacetate, succinimidyl ester (CFSE) at a final concentration of 300nM. The CFSE-labeled cells were incubated in 200 μL Xcyte media with γ-irradiated (20 Gy) splenocytes/antigen presenting cells (APCs; Balb/c or SWR/J) for 6 days. Cells were analyzed on a FACScan cytometer (BD Bioscience). For [3H]-thymidine-based MLR, [3H]-thymidine (1.0 uCi) was added on day 5 and incubated for 18 hours. Cells were analyzed using a 1450 MicroBeta TriLux microplate scintillation and luminescence counter (PerkinElmer).
Allo-HSCT
Allo-HSCT was performed as previously described.19,20 In brief, 5 × 106 T cell–depleted bone marrow cells [TCD BMs; CD45.1+ B6 (H-2b)] and 5 × 105 Tconv (CD45.2+ B6) were injected into lethally irradiated (925cGy) Balb/c recipient mice (H-2d, CD45.2+). For delayed donor lymphocyte infusions (DLI), 5 × 106 TCD BM (CD45.1+ B6) were injected into lethally irradiated (925cGy) Balb/c recipient mice, followed by injection of 2 × 106 Tconv (CD45.2+ B6) on day 11 after allo-HSCT.
Histopathology
Tissues were fixed in 10% formalin. Tissue sections of skin, liver and intestine were graded by a veterinary pathologist (Division of Comparative Medicine, Washington University School of Medicine, St Louis, MO) in blinded fashion for acute GVHD according to the Lerner grading system.21 A scale from 0 to 4 was used where histopathologic changes were identified as follows: 1, mild; 2, moderate; 3, severe; and 4, maximal. Cumulative histopathology scores from each organ were calculated.
In vivo bioluminescence imaging
CXCR3 construct
CXCR3 cDNA (clone ID: 30749408) was purchased from Open Biosystems (Thermo Fisher Scientific). A missense mutation (T > C) at nucleotide 47 found in this cDNA clone was corrected by QuikChange II XL site-directed mutagenesis kit (Agilent Technologies). The cDNA was sequenced and inserted into a CMV-based vector23 that was modified to express ires-CBRgfp.
Retroviral transduction of T cells
For the CXCR3 rescue experiments, T cells were isolated using AutoMACS and activated in the presence of anti-CD3/CD28 antibody-coated beads (bead:cell = 3:1) and human recombinant IL-2 (10 U/mL) for 24 hours before retroviral transduction. Retroviral transduction was repeated the next day. Two days after the second transduction (maximum of 4 days of in vitro activation), transduced cells were purified using the Reflection cell sorter (iCyt; GFP+ WT Tconv and GFP+ IFNγR−/− Tconv) or AutoMACS (CXCR3+ IFNγR−/− Tconv). The purity of the cells was more than 96%.
Competitive assay
WT Tconvs (5 × 105; CD45.1+ B6) and IFNγR−/− Tconvs (CD45.2+ B6; both are naive pan T cells and contain Tregs) were cotransplanted (50:50) into the same Balb/c recipient mice (H-2d, CD45.2+) one day after total body irradiation (925cGy) along with 5 × 106 TCD BM [CD45.1/CD45.2, B6 (H-2b)].
JAK1/JAK2 inhibitors
INCB018424 and CYT387 were purchased from ChemieTek and Selleck Chemicals, and prepared for injection as described in the manufacturer's instructions.
Statistical analysis
The significance of differences in survival of treatment groups was analyzed using the log-rank test. For all other analyses, the unpaired t test was used. P values < .05 were considered significant.
Results
IFNγR−/− Tconvs do not induce lethal GVHD
To determine the role of the IFNγR signaling in allogeneic Tconvs we performed MHC-mismatched allo-HSCT [B6 (H-2b)→Balb/c (H-2d)]. Mice transplanted with IFNγR−/− Tconv had improved survival, and less clinical GVHD compared with mice transplanted with WT Tconvs; both not statistically different from mice receiving T cell–depleted bone marrow (TCD BM) from B6 donors only (P = .2057; Figure 1A). Infusion of IFNγR−/− Tconvs resulted in complete donor chimerism (Figure 1B), significantly higher percentages of donor CD3+ T cells and B220+ B cells in peripheral blood (Figure 1C) and less weight loss compared with mice transplanted with WT Tconv (Figure 1D), all of which are consistent with less clinical GVHD in the IFNγR−/− Tconv recipients.20 Of interest is that mice transplanted with IFNγ−/− Tconv suffered more severe GVHD than those mice transplanted with WT Tconv (Figures 1D and 4D), which is consistent with previous reports.10-17
IFNγR−/− Tconvs do not cause life-threatening GVHD. (A) Effect of IFNγR on GVHD and survival (a pool of 4 independent experiments). Allo-HSCT (B6 [H-2b] → Balb/c]H-2d, CD45.2+]) was performed as follows. T cell–depleted bone marrow cells (TCD BMs; 5 × 106; CD45.1+ B6) and 5 × 105 Tconvs (CD45.2+ B6, either WT or IFNγR−/−) were injected into lethally irradiated (925cGy) Balb/c recipient mice. XRT: irradiation control, BM: TCD BM only, WT: TCD BM + WT Tconv, IFNγR−/−: TCD BM + IFNγR−/−Tconv, IFNγ−/−: TCD BM + IFNγ−/− Tconv. (B-D) One hundred percent donor chimerism is achieved in the IFNγR group. Peripheral blood was analyzed at day 30 after allo-HSCT. The IFNγR group shows higher CD3+ T cells and B220+ B cells in peripheral blood at day 30 after HSCT (a pool of 3 independent experiments) and better weight maintenance compared with the WT group (n = 4; one representative of 4 independent experiments). (E) Tissue sections of skin, liver, and intestine were graded by a veterinary pathologist in blinded fashion on day 20 after allo-HSCT for acute GVHD according to the Lerner grading system (see “Methods” for details).21
IFNγR−/− Tconvs do not cause life-threatening GVHD. (A) Effect of IFNγR on GVHD and survival (a pool of 4 independent experiments). Allo-HSCT (B6 [H-2b] → Balb/c]H-2d, CD45.2+]) was performed as follows. T cell–depleted bone marrow cells (TCD BMs; 5 × 106; CD45.1+ B6) and 5 × 105 Tconvs (CD45.2+ B6, either WT or IFNγR−/−) were injected into lethally irradiated (925cGy) Balb/c recipient mice. XRT: irradiation control, BM: TCD BM only, WT: TCD BM + WT Tconv, IFNγR−/−: TCD BM + IFNγR−/−Tconv, IFNγ−/−: TCD BM + IFNγ−/− Tconv. (B-D) One hundred percent donor chimerism is achieved in the IFNγR group. Peripheral blood was analyzed at day 30 after allo-HSCT. The IFNγR group shows higher CD3+ T cells and B220+ B cells in peripheral blood at day 30 after HSCT (a pool of 3 independent experiments) and better weight maintenance compared with the WT group (n = 4; one representative of 4 independent experiments). (E) Tissue sections of skin, liver, and intestine were graded by a veterinary pathologist in blinded fashion on day 20 after allo-HSCT for acute GVHD according to the Lerner grading system (see “Methods” for details).21
IFNγR−/− Tconv are defective in trafficking to GVHD target organs partially because of lack of CXCR3 expression
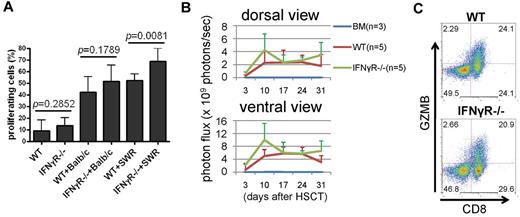
There are several possibilities underlying the defect of IFNγR−/− Tconv in induction of GVHD. These include defects in (1) allo-reactivity, (2) cytotoxicity, and/or (3) trafficking. We first examined whether IFNγR−/− Tconv were defective in allo-reactivity by performing in vitro MLRs (Figure 2A) and in vivo BLI of Tconvs that were retrovirally transduced with the click beetle red luciferase CBRluc gene (Figure 2B). We found that both their proliferation in vitro and expansion in vivo were normal and that IFNγR−/− Tconv were more proliferative than WT Tconv against the third-party APCs obtained from SWR mice (Figure 2A-B, supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These results suggest that IFNγR−/− Tconv were not defective in alloreactivity. CD8+ T cells from IFNγR−/− animals, when activated, expressed similar levels of intracellular granzyme B (GZMB), a mediator of T-cell cytotoxicity, as WT T cells (Figure 2C, supplemental Figure 2), suggesting that they are competent in killing targets. Moreover, this result is consistent with our GvL experiments discussed below (see Figure 5) showing that IFNγR−/− Tconvs were equally potent in killing allogeneic leukemia cells compared with WT Tconvs. These results suggest that the dramatic reduction of GVHD observed in the recipient mice infused with IFNγR−/− Tconv was not because of the inability of IFNγR−/− Tconvs to respond to allogeneic antigens or kill allogeneic targets.
IFNγR−/− T cells respond normally to allogeneic APCs both in vitro and in vivo. (A) IFNγR−/− Tconvs respond normally to allogeneic APC. Tconvs were labeled with CFSE and mixed lymphocyte reactions (MLRs) were performed in which 2 different strains of APC, Balb/c (H-2d) and third party SWR (H-2q) were used as stimulators. Data represent the pool of 2 independent experiments. (B) BLI was performed. The click beetle red luciferase gene (CBRluc)–expressing Tconvs (4 × 106 cells) were transplanted into Balb/c recipients. Photon flux (photons/s) was measured from the dorsal and the ventral view with a region of interest drawn over the entire body of each mouse. P values at days 10 and 31 were .1165 or higher. (C) IFNγR−/− Tconvs (bottom panel) express similar levels of GZMB, compared with WT Tconv (top panel) after 3 days of activation using anti-CD3/CD28 antibody-coated beads.
IFNγR−/− T cells respond normally to allogeneic APCs both in vitro and in vivo. (A) IFNγR−/− Tconvs respond normally to allogeneic APC. Tconvs were labeled with CFSE and mixed lymphocyte reactions (MLRs) were performed in which 2 different strains of APC, Balb/c (H-2d) and third party SWR (H-2q) were used as stimulators. Data represent the pool of 2 independent experiments. (B) BLI was performed. The click beetle red luciferase gene (CBRluc)–expressing Tconvs (4 × 106 cells) were transplanted into Balb/c recipients. Photon flux (photons/s) was measured from the dorsal and the ventral view with a region of interest drawn over the entire body of each mouse. P values at days 10 and 31 were .1165 or higher. (C) IFNγR−/− Tconvs (bottom panel) express similar levels of GZMB, compared with WT Tconv (top panel) after 3 days of activation using anti-CD3/CD28 antibody-coated beads.
We next hypothesized that IFNγR−/− Tconvs might migrate differently from WT Tconvs to GVHD target organs. By performing BLI, we found that IFNγR−/− Tconvs trafficked primarily to the spleen, whereas WT Tconvs migrated primarily to the GI tract and lymph nodes (LNs; Figure 3A) and that IFNγR expression was highly induced on the WT Tconv surface when activated (Figure 3B). To identify possible chemokine receptors that were regulated by IFNγR, we performed FACS for all chemokine receptors for which commercially available antibodies existed including CXCR2, CXCR3, CXCR4, CXCR5, CCR3, CCR4, CCR5, CCR6, CCR7, and CCR9 (supplemental Figure 3). We also examined IL-21R expression, based on recent data reported by Hanash et al24 (supplemental Figure 4). We found that CXCR3 was the only chemokine receptor tested that was differentially expressed in bead-activated WT Tconvs compared with IFNγR−/− Tconvs (Figure 3B-C, supplemental Figure 5). We further found that expression of IFNγR and CXCR3 were coordinately up-regulated in WT Tconvs after bead activation (Figure 3B). In addition, we found that 20%-25% of Tconvs at resting state were CXCR3+ (preactivation; Figure 3B-C) and that these CXCR3+ naive Tconvs were primarily CD44+ memory T cells25 (supplemental Figure 6). Infusion of sorted and purified CD44+ central memory (CM) T cells have been shown by others to not induce detectable GVHD in MHC-mismatched allogeneic transplant recipient mice.25
IFNγR is required for trafficking of Tconvs to GVHD target organs and CXCR3 expression. (A) In vivo BLI was performed to specifically track CBRluc-transduced Tconvs (2 or 4 × 106 cells) after allo-HSCT. BLI images of 1 dissected representative mouse from WT (left) and IFNγR−/− (right) T cell recipients at day 31 after allo-HSCT (top panels). Spleens and GI tracts were separated from the body cavities. White arrows indicate LNs. Ratio of signal intensities (photons/s/cm2/sr) from spleen and GI tract and the rest of body were compared in the bottom panel. Data represent the pool of 2 independent experiments. (B) WT Tconvs express IFNγR (CD119) and CXCR3 before (top panels) and after (bottom panels) activation by anti-CD3/CD28 antibody-coated beads. The expressions of IFNγR (CD119) and CXCR3 are correlated in WT Tconv. (C) IFNγR−/− Tconv (both CD4+ and CD8+ T cells; CD4− T cells are CD8+ T cells right panels) express CXCR3 significantly less than WT Tconvs (left panels) after activation (bottom panels). Mean and SD of activated WT Tconvs are as follows (n = 8). CD8+CXCR3+: 42.7% ± 5.5%, CD8+CXCR3−: 5.2% ± 1.3%, CD4+CXCR3+: 41.2% ± 5.0%, CD4+CXCR3−: 11.0% ± 4.7%. Mean and standard deviation of activated IFNγR−/− Tconv are as follows (n = 8). CD8+CXCR3+: 24.1% ± 8.2%, CD8+CXCR3−: 21.0% ± 3.3%, CD4+CXCR3+: 5.5% ± 1.7%, and CD4+CXCR3−: 49.3% ± 4.8%. (D) IFNγ−/− Tconv (both CD4+ and CD8+ T cells; CD4− T cells are CD8+ T cells) express CXCR3 significantly less than WT Tconvs (C) after activation (bottom panels) but up-regulate IFNγR expression similar to activated WT Tconvs (left panels). Mean and standard deviation of activated IFNγ−/− Tconv are as follows (n = 9). CD8+CXCR3+: 23.4% ± 5.3%, CD8+CXCR3−: 24.6% ± 4.9%, CD4+CXCR3+: 5.6% ± 2.6%, and CD4+CXCR3−: 46.4% ± 6.9%. (E) IFNγR−/− Tconvs were retrovirally transduced with either the CBRluc-GFP gene (IFNγR−/−) or the CXCR3-ires-CBRluc-GFP gene (CXCR3+ IFNγR−/−). WT Tconvs were also transduced with the CBRluc-GFP gene. Transduced cells were purified using the Reflection cell sorter (iCyt; GFP+ WT Tconvs and GFP+ IFNγR−/− Tconvs) or AutoMACS (CXCR3+ IFNγR−/− Tconvs; all cells > 96% pure) and transplanted (2 × 106) into recipient mice along with TCD BM (5 × 106). Shown is percent survival after allo-HSCT (5 × 106 TCD BM + 2 × 106 Tconv). Data represent a pool of 3 independent experiments.
IFNγR is required for trafficking of Tconvs to GVHD target organs and CXCR3 expression. (A) In vivo BLI was performed to specifically track CBRluc-transduced Tconvs (2 or 4 × 106 cells) after allo-HSCT. BLI images of 1 dissected representative mouse from WT (left) and IFNγR−/− (right) T cell recipients at day 31 after allo-HSCT (top panels). Spleens and GI tracts were separated from the body cavities. White arrows indicate LNs. Ratio of signal intensities (photons/s/cm2/sr) from spleen and GI tract and the rest of body were compared in the bottom panel. Data represent the pool of 2 independent experiments. (B) WT Tconvs express IFNγR (CD119) and CXCR3 before (top panels) and after (bottom panels) activation by anti-CD3/CD28 antibody-coated beads. The expressions of IFNγR (CD119) and CXCR3 are correlated in WT Tconv. (C) IFNγR−/− Tconv (both CD4+ and CD8+ T cells; CD4− T cells are CD8+ T cells right panels) express CXCR3 significantly less than WT Tconvs (left panels) after activation (bottom panels). Mean and SD of activated WT Tconvs are as follows (n = 8). CD8+CXCR3+: 42.7% ± 5.5%, CD8+CXCR3−: 5.2% ± 1.3%, CD4+CXCR3+: 41.2% ± 5.0%, CD4+CXCR3−: 11.0% ± 4.7%. Mean and standard deviation of activated IFNγR−/− Tconv are as follows (n = 8). CD8+CXCR3+: 24.1% ± 8.2%, CD8+CXCR3−: 21.0% ± 3.3%, CD4+CXCR3+: 5.5% ± 1.7%, and CD4+CXCR3−: 49.3% ± 4.8%. (D) IFNγ−/− Tconv (both CD4+ and CD8+ T cells; CD4− T cells are CD8+ T cells) express CXCR3 significantly less than WT Tconvs (C) after activation (bottom panels) but up-regulate IFNγR expression similar to activated WT Tconvs (left panels). Mean and standard deviation of activated IFNγ−/− Tconv are as follows (n = 9). CD8+CXCR3+: 23.4% ± 5.3%, CD8+CXCR3−: 24.6% ± 4.9%, CD4+CXCR3+: 5.6% ± 2.6%, and CD4+CXCR3−: 46.4% ± 6.9%. (E) IFNγR−/− Tconvs were retrovirally transduced with either the CBRluc-GFP gene (IFNγR−/−) or the CXCR3-ires-CBRluc-GFP gene (CXCR3+ IFNγR−/−). WT Tconvs were also transduced with the CBRluc-GFP gene. Transduced cells were purified using the Reflection cell sorter (iCyt; GFP+ WT Tconvs and GFP+ IFNγR−/− Tconvs) or AutoMACS (CXCR3+ IFNγR−/− Tconvs; all cells > 96% pure) and transplanted (2 × 106) into recipient mice along with TCD BM (5 × 106). Shown is percent survival after allo-HSCT (5 × 106 TCD BM + 2 × 106 Tconv). Data represent a pool of 3 independent experiments.
Because T cells were the only source of IFNγ in this assay, we hypothesized that up-regulation of CXCR3 in IFNγ−/− Tconvs would be blunted compared with WT Tconvs. We indeed observed that bead-activated IFNγ−/− Tconvs (compared with WT Tconvs) expressed the same reduced level of CXCR3 as activated IFNγR−/− Tconv. Of note, the addition of exogenous IFNγ to cultures of IFNγ−/− Tconvs rescued CXCR3 expression in bead-activated IFNγ−/− Tconvs to the same level as WT Tconvs (supplemental Figure 7). These data suggest that CXCR3 expression is positively regulated or maintained by IFNγ via IFNγR signaling (Figure 3B-D). If IFNγR-induced up-regulation of CXCR3 was the primary mediator of Tconv trafficking to GVHD organs and clinical GVHD then overexpression of CXCR3 in IFNγR−/− would partially or completely rescue the GVHD defect seen in IFNγR−/− Tconvs. Although there was a trend toward enhanced GVHD in IFNγR−/− Tconvs transduced with CXCR3 (Figure 3E), the effects were not statistically significant using a major-mismatched transplant model (B6 to Balb/c). These data suggest that other molecules and pathways regulated by IFNγR signaling are critical for T-cell trafficking and GVHD. The effects of CXCR3 expression on GVHD in minor-mismatched models is more obvious (see “Discussion” and supplemental Figure 9).
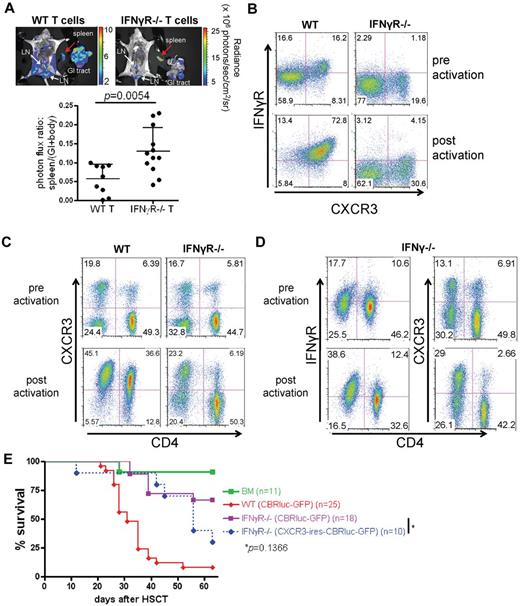
Tregs also require IFNγR for suppressor function in vivo
Because Tregs express both IFNγR and CXCR3 when activated (Figure 4A), we hypothesized that Tregs also require the IFNγR signaling for proper trafficking. If our hypothesis is correct that IFNγR signaling is necessary for Treg migration to the GVHD target organs, it is expected that IFNγR−/− Tregs will function in vitro but will not suppress GVHD in vivo when cotransplanted with alloreactive Tconv in allo-HSCT settings because of defective trafficking. Likewise, it is expected that when WT Tregs are transplanted along with IFNγ−/− Tconv, there will be a failure to suppress GVHD because the major source of IFNγ for Tregs is Tconvs.26 We found that IFNγR−/− Tregs were equally suppressive as WT Tregs in vitro (Figure 4B) but in contrast to WT Tregs, could not suppress GVHD in vivo (Figure 4C). In addition, WT Tregs suppressed GVHD induced by WT Tconvs but not by IFNγ−/− Tconvs (Figure 4D), indicating that Tregs require donor T cell-derived IFNγ for proper suppressor function in vivo. These results suggest that the IFNγR signaling might be required for Treg trafficking to GVHD target organs (see Figure 6). These data may explain why IFNγ−/− Tconvs cause more severe GVHD than WT Tconvs (anti-inflammatory effect10-14 ; Figures 1D and 4D). Likewise, our model may provide an explanation for the observed proinflammatory effects of IFNγ in that reduced levels of IFNγ in local sites of inflammation or GVHD leads to reduced trafficking of activated T cells to these sites and less severe GVHD.
IFNγR is required for Tregs to function appropriately in vivo. (A) Tregs also up-regulate both CXCR3 and IFNγR when activated (right panels). Mean and standard deviation of activated Tregs are as follows (n = 3). IFNγR+CXCR3−: 16.8% ± 7.0%, IFNγR-CXCR3−: 1.39% ± 1.2%, IFNγR+CXCR3+: 79.1% ± 6.1%, and IFNγR-CXCR3+: 2.7% ± 1.8%. (B) IFNγR Tregs were equally suppressive as WT Tregs in in vitro MLR assays ([3H]-thymidine incorporation was measured). Tregs were serial-diluted. The experiment was performed in triplicate or quadruplicate. Shown is 1 representative of 3 independent experiments with similar results. (C) IFNγR−/− Tregs do not suppress GVHD (B6 → Balb/c). TCD BM (B6, 5 × 106; CD45.1+) were injected into lethally irradiated Balb/c mice, followed by DLI of 2 × 106 pan T (B6, CD45.2+) and 1.5 × 106 Tregs (B6, CD45.2+). Data represent the pool of 2 independent experiments. (D) WT Tregs suppress GVHD induced by WT Tconvs but not by IFNγ−/− Tconv. Both Tregs and Tconvs (5 × 105 cells each) were injected along with TCD BM (5 × 106 cells) at day 0. Data represent the pool of 2 independent experiments.
IFNγR is required for Tregs to function appropriately in vivo. (A) Tregs also up-regulate both CXCR3 and IFNγR when activated (right panels). Mean and standard deviation of activated Tregs are as follows (n = 3). IFNγR+CXCR3−: 16.8% ± 7.0%, IFNγR-CXCR3−: 1.39% ± 1.2%, IFNγR+CXCR3+: 79.1% ± 6.1%, and IFNγR-CXCR3+: 2.7% ± 1.8%. (B) IFNγR Tregs were equally suppressive as WT Tregs in in vitro MLR assays ([3H]-thymidine incorporation was measured). Tregs were serial-diluted. The experiment was performed in triplicate or quadruplicate. Shown is 1 representative of 3 independent experiments with similar results. (C) IFNγR−/− Tregs do not suppress GVHD (B6 → Balb/c). TCD BM (B6, 5 × 106; CD45.1+) were injected into lethally irradiated Balb/c mice, followed by DLI of 2 × 106 pan T (B6, CD45.2+) and 1.5 × 106 Tregs (B6, CD45.2+). Data represent the pool of 2 independent experiments. (D) WT Tregs suppress GVHD induced by WT Tconvs but not by IFNγ−/− Tconv. Both Tregs and Tconvs (5 × 105 cells each) were injected along with TCD BM (5 × 106 cells) at day 0. Data represent the pool of 2 independent experiments.
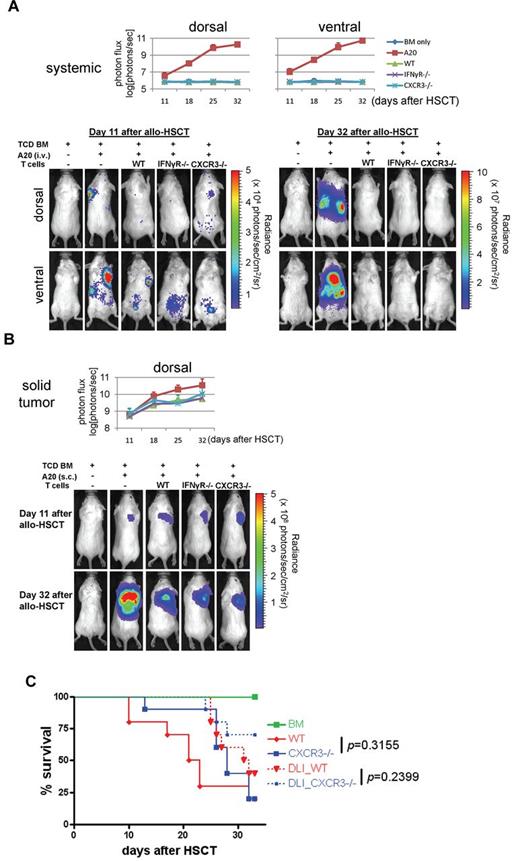
IFNγR−/− Tconvs induce a strong GvL/GvT effect
The clinical goal of allo-HSCT is to minimize GVHD and maximize GvL. To test whether IFNγR−/− Tconv maintain a robust GvL, we performed BLIs using 2 different mouse models of leukemia; one a disseminated leukemia model20 (Figure 5A) and the other a solid tumor lymphoma model (Figure 5B). For the leukemia model, CBRluc-expressing A20 leukemia cells (Balb/c-derived) were transplanted intravenously (1 × 104 cells) along with TCD BM (B6, CD45.1+) and Tconvs (B6, CD45.2+) either from WT or IFNγR−/− mice into Balb/c recipients at day 0. In fact, IFNγR−/− Tconvs were as effective as WT Tconvs at eliminating all detectable A20 cells by day 32 after allo-HSCT. For the solid tumor model, the same CBRluc-expressing A20 cells were injected subcutaneously (1 × 105 cells) at the shoulder and TCD BM intravenously into Balb/c recipients at day 0. The A20 cells grew primarily as a solitary tumor nodule. Allogeneic Tconvs either from WT or IFNγR−/− mice were injected as DLI at day 11. We found that IFNγR−/− Tconvs, similar to WT Tconvs, mediated a potent GvT effect. Of note is that a similar robust GvL/GvT effect was observed with CXCR3−/− Tconvs (Figure 5). These data suggest that genetic or pharmacologic modulation of the IFNγR signaling would be a promising therapeutic approach to overcome HLA-barrier in allo-HSCT.
IFNγR−/− and CXCR3−/− Tconvs mediate a robust GvL/GvT. (A) Systemic leukemia model. Photon flux was measured with a region of interest drawn over the entire body of each mouse. Actual images of 1 representative mouse from each group are shown in bottom panels. Data represent the pool of 2 independent experiments. n = 10 each group (n = 6 in BM only group). (B) Solid tumor model. Photon flux was measured with a region of interest drawn over the entire body of each mouse. Actual images of 1 representative mouse from each group are shown in bottom panels. Data represent the pool of 2 independent experiments. n = 10 each group (n = 6 in BM only group). (C) Effect of CXCR3 on GVHD and survival. Data represent the pool of 2 independent experiments (n = 10).
IFNγR−/− and CXCR3−/− Tconvs mediate a robust GvL/GvT. (A) Systemic leukemia model. Photon flux was measured with a region of interest drawn over the entire body of each mouse. Actual images of 1 representative mouse from each group are shown in bottom panels. Data represent the pool of 2 independent experiments. n = 10 each group (n = 6 in BM only group). (B) Solid tumor model. Photon flux was measured with a region of interest drawn over the entire body of each mouse. Actual images of 1 representative mouse from each group are shown in bottom panels. Data represent the pool of 2 independent experiments. n = 10 each group (n = 6 in BM only group). (C) Effect of CXCR3 on GVHD and survival. Data represent the pool of 2 independent experiments (n = 10).
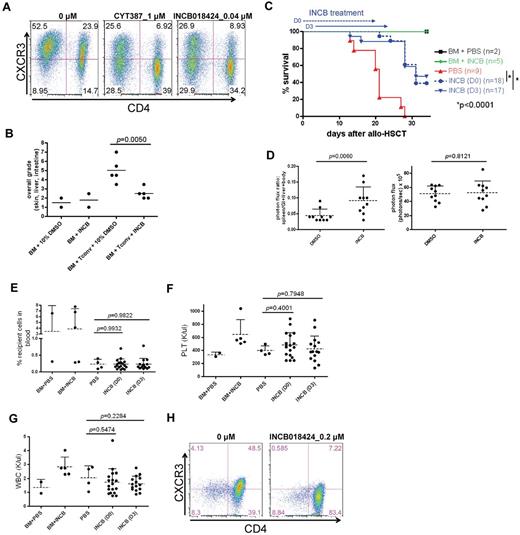
IFNγR signaling inhibitors prevent CXCR3 expression and GVHD
IFNγR signaling is mediated via JAK1 and JAK2. Therefore, we hypothesized that JAK1/JAK2–specific inhibitors will block IFNγR-mediated CXCR3 expression and thus GVHD. CYT387 and INCB018424 are commercially available potent JAK1/JAK2–specific inhibitors.27,28 Both are currently being tested in clinical trials for the treatment of myeloproliferative disorders and INCB018424 was recently approved by FDA for the treatment of patients with high risk myelofibrosis. These small molecule inhibitors dramatically reduced the expression of CXCR3 in activated WT T cells (Figure 6A). Of particular interest is that the effects of these JAK1/JAK2 inhibitors on CXCR3 expression in WT T cells exactly phenocopy the decreased expression of CXCR3 seen in activated IFNγR−/− T cells (Figures 6A and 3C). Moreover, in vivo administration of INCB018424 to mice transplanted with WT Tconvs significantly reduced GVHD (Figure 6B) and improved survival (Figure 6C). Most importantly, this drug alters T-cell trafficking (Figure 6D left panel) similarly as seen in the IFNγR−/− T-cell recipients (Figure 3A), although not affecting T-cell expansion (Figure 6D right panel). Of interest is that there was limited mortality and weight loss (data not shown) during the period of drug administration suggesting that continued administration of these JAK1/JAK2 inhibitors chronically after transplant may provide long-term protection against GVHD while maintaining a strong GvL effect of alloreactive donor T cells. In addition, we saw no obvious effect of daily administration of INCB018424 after allo-HSCT on donor engraftment or blood counts (Figure 6E-G), suggesting that continued and daily administration in humans after transplant may be associated with negligible or limited hematopoietic toxicity. Finally, INCB018424 potently inhibited CXCR3 expression in CD3/CD28 activated human T cells as well (Figure 6H), suggesting that human CXCR3 is also regulated by IFNγR signaling pathway and that human GVHD could potentially be mitigated through targeting of IFNγR signaling.
JAK1/JAK2 inhibitors block the IFNγR-CXCR3 axis both in mouse and human T cells and mitigates GVHD. (A) Shown are WT pan T cells (both CD4+ and CD8+ T cells; CD4− T cells are CD8+ T cells) after drug treatment. (B) Tissue sections of skin, liver, and intestine were graded by a veterinary pathologist in blinded fashion on day 21 after allo-HSCT for acute GVHD according to the Lerner grading system (see “Methods” for details).21 (C) Effect of INCB018424 on GVHD. Allo-HSCT (B6 (H-2b) → Balb/c (H-2d) was performed as follows. Five × 106 T cell–depleted bone marrow cells (TCD BM) and 5 × 105 Tconvs were injected into lethally irradiated (925cGy) Balb/c recipient mice. INCB018424 was injected intraperitoneally into recipients daily from day 0 through 20 (D0: 100 μg twice daily for the first 7 days and 100 μg once daily for the following 14 days) or day 3 through 23 (D3: 100 μg twice daily for the first 4 days and 100 μg once daily for the following 17 days). (D) In vivo BLI was performed to specifically track T cells (0.5 × 106 cells) obtained from FVB-Tg(CAG-luc,-GFP)L2G85Chco/J mice (H-2q) after allo-HSCT. FVB/NJ mice (H-2q) were used as TCD BM donors (5 × 106 cells) and Balb/c as recipients. INCB018424 or 10% DMSO was administered twice a day from days 3 to 6 and once daily from days 7 to 23. BLI images of dissected mice (n = 10 each) at day 31 after allo-HSCT were analyzed. Spleens, livers, and GI tracts were separated from the body cavities. Photon flux (photons/s) was measured from whole body (right panel) and the ratio of signal intensities (photons/s/cm2/sr) from spleen, liver, and GI tract and the rest of body were compared (left panel). (E-G) INCB018424 does not inhibit donor engraftment and reduce neither platelet (PLT) counts nor white blood cell (WBC) counts. Day 21 after allo-HSCT, BM+PBS (n = 2), BM+INCB (n = 5), PBS (n = 4), INCB (D0; n = 18), and INCB (D3; n = 15). (H) Shown are human pan T cells 5 days after drug treatment in the presence of anti-CD3/CD28 antibody coated beads (cell:bead = 1:1). Mean and SD of activated human T cells are as follows (n = 2); 0μM of INCB018424: CD8+CXCR3+: 6.0% ± 2.7%, CD8+CXCR3−: 6.3% ± 2.8%, CD4+CXCR3+: 49.6% ± 1.6%, CD4+CXCR3−: 38.1% ± 1.5%. 0.2μM of INCB018424: CD8+CXCR3+: 0.4% ± 0.2%, CD8+CXCR3−: 8.7% ± 0.3%, CD4+CXCR3+: 10.2% ± 4.2%, and CD4+CXCR3−: 80.8% ± 3.7%.
JAK1/JAK2 inhibitors block the IFNγR-CXCR3 axis both in mouse and human T cells and mitigates GVHD. (A) Shown are WT pan T cells (both CD4+ and CD8+ T cells; CD4− T cells are CD8+ T cells) after drug treatment. (B) Tissue sections of skin, liver, and intestine were graded by a veterinary pathologist in blinded fashion on day 21 after allo-HSCT for acute GVHD according to the Lerner grading system (see “Methods” for details).21 (C) Effect of INCB018424 on GVHD. Allo-HSCT (B6 (H-2b) → Balb/c (H-2d) was performed as follows. Five × 106 T cell–depleted bone marrow cells (TCD BM) and 5 × 105 Tconvs were injected into lethally irradiated (925cGy) Balb/c recipient mice. INCB018424 was injected intraperitoneally into recipients daily from day 0 through 20 (D0: 100 μg twice daily for the first 7 days and 100 μg once daily for the following 14 days) or day 3 through 23 (D3: 100 μg twice daily for the first 4 days and 100 μg once daily for the following 17 days). (D) In vivo BLI was performed to specifically track T cells (0.5 × 106 cells) obtained from FVB-Tg(CAG-luc,-GFP)L2G85Chco/J mice (H-2q) after allo-HSCT. FVB/NJ mice (H-2q) were used as TCD BM donors (5 × 106 cells) and Balb/c as recipients. INCB018424 or 10% DMSO was administered twice a day from days 3 to 6 and once daily from days 7 to 23. BLI images of dissected mice (n = 10 each) at day 31 after allo-HSCT were analyzed. Spleens, livers, and GI tracts were separated from the body cavities. Photon flux (photons/s) was measured from whole body (right panel) and the ratio of signal intensities (photons/s/cm2/sr) from spleen, liver, and GI tract and the rest of body were compared (left panel). (E-G) INCB018424 does not inhibit donor engraftment and reduce neither platelet (PLT) counts nor white blood cell (WBC) counts. Day 21 after allo-HSCT, BM+PBS (n = 2), BM+INCB (n = 5), PBS (n = 4), INCB (D0; n = 18), and INCB (D3; n = 15). (H) Shown are human pan T cells 5 days after drug treatment in the presence of anti-CD3/CD28 antibody coated beads (cell:bead = 1:1). Mean and SD of activated human T cells are as follows (n = 2); 0μM of INCB018424: CD8+CXCR3+: 6.0% ± 2.7%, CD8+CXCR3−: 6.3% ± 2.8%, CD4+CXCR3+: 49.6% ± 1.6%, CD4+CXCR3−: 38.1% ± 1.5%. 0.2μM of INCB018424: CD8+CXCR3+: 0.4% ± 0.2%, CD8+CXCR3−: 8.7% ± 0.3%, CD4+CXCR3+: 10.2% ± 4.2%, and CD4+CXCR3−: 80.8% ± 3.7%.
Discussion
The clinical goal of allo-HSCT is to eliminate GVHD while maintaining GvL. In this study, we demonstrated that IFNγR positively regulates the expression of chemokine receptor CXCR3 that is partially responsible for alloreactive donor T cells trafficking to GVHD target organs. In addition, we show that IFNγR−/− T cells are defective in causing GVHD while maintaining a beneficial GvL/GvT effect. Based on the data presented, we hypothesize that T-cell activation by allo-APCs leads to the expression of IFNγ and IFNγR in activated Tconvs. Subsequently, IFNγR signaling induces CXCR3 expression. This, in part, promotes the migration of alloreactive Tconvs to GVHD target organs secreting a gradient of CXCR3-ligands, CXCL9, CXCL10, and CXCL11 (CXCL9-11; Figure 7), which are also regulated by IFNγR.29 Therefore, it is expected that inhibition of IFNγR signaling will result in reduction of both CXCR3 and CXCL9-11 in donor Tconvs and in recipient tissues, respectively, thereby decreasing T-cell trafficking to GVHD target organs and GVHD. However, migration of T cells to BM, spleen, blood, and other sites where leukemic cells accumulate will not be affected. Therefore, the IFNγR signaling pathway represents a promising therapeutic target for altering the trafficking of allogeneic Tconv and mitigating GVHD while preserving the GvL effect.
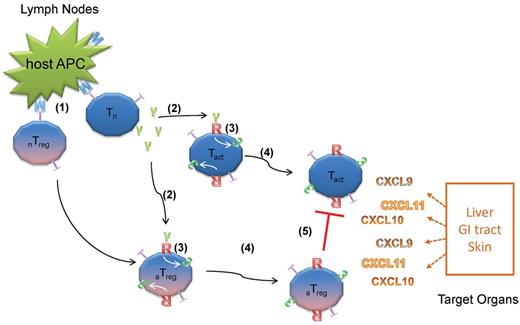
Model for the role of IFNγR signaling in T-cell trafficking and GVHD. Naive Tconvs and Tregs become activated by host APCs because of MHC mismatch. (1) Activated Tconv secrete IFNγ (2) that will initiate the IFNγR signaling, which in turn up-regulates CXCR3 and other unknown mediators of T-cell trafficking on both Tconvs and Tregs. (3) CXCR3+ T cells migrate to the CXCR3 ligands-expressing GVHD target organs (4) where activated Tconv are destructive but suppressed in the presence of activated Tregs. (5) When Tconvs fail to secrete IFNγ (IFNγ−/− Tconv), Tregs will not activate IFNγR signaling, thereby failing to migrate to the same sites of inflammation as IFNγ−/− Tconv. This will result in more severe GVHD than WT Tconv. Likewise, when Tregs are defective in up-regulating CXCR3 because of the lack of IFNγR, the same result will be observed. Tn: naive Tconv, Tact: activated Tconv, nTreg: naive Tregs, aTreg: activated Tregs, M: MHC molecules, T: T-cell receptors, R: IFNγR, 3: CXCR3, γ: IFNγ.
Model for the role of IFNγR signaling in T-cell trafficking and GVHD. Naive Tconvs and Tregs become activated by host APCs because of MHC mismatch. (1) Activated Tconv secrete IFNγ (2) that will initiate the IFNγR signaling, which in turn up-regulates CXCR3 and other unknown mediators of T-cell trafficking on both Tconvs and Tregs. (3) CXCR3+ T cells migrate to the CXCR3 ligands-expressing GVHD target organs (4) where activated Tconv are destructive but suppressed in the presence of activated Tregs. (5) When Tconvs fail to secrete IFNγ (IFNγ−/− Tconv), Tregs will not activate IFNγR signaling, thereby failing to migrate to the same sites of inflammation as IFNγ−/− Tconv. This will result in more severe GVHD than WT Tconv. Likewise, when Tregs are defective in up-regulating CXCR3 because of the lack of IFNγR, the same result will be observed. Tn: naive Tconv, Tact: activated Tconv, nTreg: naive Tregs, aTreg: activated Tregs, M: MHC molecules, T: T-cell receptors, R: IFNγR, 3: CXCR3, γ: IFNγ.
We also report that Tregs use similar signaling pathways for their trafficking to GVHD target organs and that Treg-trafficking to GVHD target organs depends on donor T cell–derived IFNγ (Figures 4D and 7), indicating that trafficking of both Tconvs and Tregs is tightly linked. This common pathway allows Tregs to migrate to the same sites of inflammation as alloreactive Tconvs. This is consistent with the major mechanism of Treg suppressor function; specifically that it is cell–cell contact or “close proximity” dependent.20,30-32 In addition, the requirement of CXCR3 expression in Tregs is consistent with the recent report that Tregs transduced to overexpress CXCR3 more efficiently suppressed GVHD than nontransduced Tregs.33 Indeed, we observed a different pattern of distribution of WT Tregs compared with IFNγR−/− Tregs in GVHD target organs when WT Tconv and IFNγR−/− Tconv (both are naive pan T cells and contain Tregs) were cotransplanted (50:50) into the same recipients (supplemental Figure 8), suggesting that IFNγR signaling regulates Treg trafficking to GVHD target organs.
Although our BLI studies demonstrate that T-cell trafficking to the GVHD target organs is regulated by IFNγR signaling, there are certain limitations in the interpretation of these experiments because retroviral transduction of T cells involves T-cell activation and activated T cells might behave and traffic differently from naive T cells. All of our in vitro T-cell transduction studies adhered strictly to the conditions outlined in “Methods” that we have previously shown to result in only a negligible impact on the alloreactivity and GVHD potential of activated T cells versus resting donor T cells.19,20,23
The effect of CXCR3 deficiency on GVHD is more obvious in our minor-mismatched models than in our major-mismatched model (Figure 5C, supplemental Figure 9) and is consistent with the results of the Ferrara group in which CXCR3−/− Tconvs resulted in less GVHD in minor-mismatched transplant models.34 In addition, He et al demonstrated that the treatment with blocking antibody of CXCR3 significantly reduced GVHD in minor histocompatibility antigen-mismatched GVHD models.35 Although CXCR3−/− Tconv induced less GVHD than WT Tconvs, they can still cause lethal GVHD in our transplant model (Figure 5C, supplemental Figure 9). This result and our data described in Figure 3E suggest that reduced CXCR3 expression may not be the only reason why IFNγR−/− Tconvs mediate less GVHD. Iwasaki et al proposed that WT T cells up-regulate CCR5 in the presence of IL-12 in vitro, whereas IFNγ−/− T cells express approximately 50% less after exposure to IL-12.36 Although we generated similar data, the levels of CCR5 expression were low (data not shown). More importantly, there was no significant difference in CCR5 expression between WT and IFNγ−/− T cells in vivo (data not shown). Moreover, it is not clear whether these small differences in CCR5 expression could potentially contribute to the differences in T-cell trafficking and GVHD that we observed between WT and IFNγR−/− T cells. However, we cannot rule out the possibility that a dynamic regulation of CCR5 and CXCR3 expression by IFNγR signaling is critical in vivo.
Because IFNγ is an important cytokine for normal differentiation of T cells into T helper 1 (Th1) cells whose alloreactivity is a major cause of GVHD, lack of the IFNγR signaling might lead to preferential T-cell differentiation to Th2 cells rather than Th1 cells.14,37 Although, it is possible that IFNγR−/− T cells might preferentially differentiate into Th2 rather than Th1, thereby causing less GVHD, we found that IFNγR−/− T cells, when activated with anti-CD3/CD28 beads, secreted Th1 and Th2 cytokines, such as IL-2, IFNγ, TNFα, IL-4, and IL-5, at similar levels to WT T cells, based on a cytokine ELISA (data not shown; cytokines in cell culture supernatants were analyzed [Rodent Map v2.0, Rules Based Medicine]). In addition, our in vivo competitive assay also suggests that IFNγR−/− T cells are equally capable of differentiating into Th1 cells (and Th2 and Th17 cells as well; supplemental Figure 10), which is in agreement with the previous reports that IFNγR−/− T cells can normally differentiate into IFNγ-producing Th1 cells and IL-17+ Th17 cells.38,39 Because the ratio of Tregs to non-Tregs is inversely correlated with GVHD severity,20 we also measured the ratio of donor T cell–derived CD4+FOXP3+ Tregs to CD4+FOXP3− T cells in the peripheral blood from mice transplanted with either WT or IFNγR−/− Tconvs on day 30 after allo-HSCT and found no statistical difference between the groups (P = .1063, n = 5). Alternatively, it is also possible the IFNγR−/− Tconvs secrete more Treg-specific chemokines/chemo attractants. Of note is that we found that IFNγR−/− Tconv secreted 2.5-fold more CCL22 (aka, macrophage-derived chemokine, a CCR4 ligand) than WT T cells when activated with anti-CD3/CD28 beads in vitro (2090 pg/mL versus 841 pg/mL; Rodent Map v2.0, Rules Based Medicine). This ligand has been shown to be involved in T-cell trafficking to liver and skin29,40,41 and its receptor CCR4 is preferentially overexpressed in liver infiltrating Tregs compared with non-Tregs.41 Therefore, it is possible that IFNγR−/− T cells recruit more Tregs to sites of inflammation (sites of GVHD) than WT T cells do. Finally, it is certainly possible that IFNγR signaling regulates the expression of other chemokine receptor(s) or adhesion molecules that contribute to T-cell trafficking to GVHD target organs. If so, targeted inhibition of IFNγR signaling may be a better approach to limit GVHD while preserving GvL than direct targeting of CXCR3.
Burman et al demonstrated that IFNγ inhibits allogeneic donor T-cell trafficking to the lungs and that IFNγ−/− T cells cause idiopathic pneumonia syndrome after allo-HSCT.14 Thus, we examined whether IFNγR−/− T cells show similar results after transplantation. We observed minimal, multifocal perivascular accumulation of lymphocytes in the lungs of WT T-cell recipients (80%, n = 5) whereas we observed only a minimal interstitial infiltration of neutrophils into the lungs of IFNγR−/− T-cell recipients (40%, n = 5) with no sign of lymphocyte infiltration into the lungs at day 20 after allo-HSCT (data not shown).
Wysocki et al compared the effect of CCR5 expression in donor T cells on GVHD in lethally irradiated and nonirradiated recipients using a parent to F1 (B6 to B6D2) HSCT model.42 They demonstrated that CCR5−/− T cells worsened GVHD in lethally irradiated recipients but reduced GVHD in nonirradiated ones, suggesting that the function of CCR5 is affected by the intensity of conditioning.42 Because elimination of irradiation leads to graft failure or graft rejection in our major-mismatched model, DLI was performed on day +11 after HSCT. We believe that this simulates effect of reduced intensity of irradiation. We found that there was no differential effect of delayed infusion of IFNγR−/− T cells in this model and that IFNγR−/− T cells resulted in significantly less GVHD and improved overall survival compared with WT T cells (data not shown).
Considering that the skin is a GVHD target tissue, it is surprising that infusion of resting IFNγR−/− T cells effectively reduced the subcutaneous A20 tumor growth. It is possible that A20 cells secrete chemokines that attract alloreactive donor T cells independently of the IFNγR-CXCR3 axis. It is also possible that A20 cells are highly sensitive to alloreactive T cells and this antitumor effect in the skin requires minimal number of donor T cells.
Based on our data, we administered JAK1/JAK2 inhibitors to T cells in vitro and to mice transplanted with allogeneic WT T cells in vivo. JAK1/JAK2 inhibitors (INCB018424 and CYT387) successfully blocked the up-regulation of CXCR3 expression after bead activation of both murine and human T cells in vitro exactly phenocopying what we observed with murine IFNγR−/− Tconv. In addition, in vivo administration of INCB018424 altered the trafficking of allogeneic T cells and significantly reduced lethal GVHD after allo-HSCT. Although our study demonstrated that the IFNγR signaling pathway represents a promising therapeutic target for mitigating GVHD and provides the foundation for future clinical trials using these reagents as prophylaxis and treatment of GVHD in humans, it still remains to be determined whether JAK1/JAK2 inhibitors maintain GvL. In addition, because CXCL9-11 are also regulated by the IFNγR signaling pathway,29 it is possible that administration of JAK1/JAK2 inhibitors results in a reduction of CXCL9-11 production from GVHD target organs, thereby attracting less of alloreactive donor T cells. Alternatively, JAK1/JAK2 inhibitors might reduce T-cell response to allo-APCs possibly by inhibiting signaling via proinflammatory cytokines such as IL-6, IL-12, and IL-23, which are important for Th1 and Th17 differentiation, as Betts et al demonstrated in in vitro MLRs using JAK2 inhibitor.43 Lastly, although Ma et al did not investigate a possibility of altered trafficking of STAT1−/− T cells to GVHD target organs, they recently reported that deficiency in STAT1 signaling in allogeneic donor T cells resulted in reduction of GVHD, enhancing the survival and expansion of natural Tregs and generation of inducible Tregs.44 Therefore, it is possible that JAK1/JAK2 inhibitors might mitigate GVHD by increasing Tregs.
In conclusion, IFNγR signaling plays a major role in T-cell trafficking to GVHD target organs partially via CXCR3. Inhibition of the IFNγR signaling pathway in allogeneic Tconv results in reduced GVHD while maintaining a robust GvL effect. Interestingly, this same pathway appears to be critical for Treg trafficking and function in vivo as well. Thus, we propose that the IFNγR signaling pathway, including IFNγR, JAK1/JAK2, STAT1, T-bet (Th1-specific transcription factor that regulates CXCR3 expression), and CXCR3, might be promising therapeutic targets in allo-HSCT. In addition, modulation of CXCR3 ligands CXCL9-11 expression in allo-HSCT recipients could likewise affect the severity of GVHD without necessarily altering GvL activity. Finally, polymorphisms in the IFNγR, CXCR3, and CXCL9-11genes were previously shown to be associated with the severity of infectious diseases, such as hepatitis and tuberculosis, and inflammatory disorders (inflammatory bowel disease, asthma, and coronary disease).45-51 Therefore, correlation of polymorphisms in IFNγR-CXCR3-CXCL9-11 axis genes with GVHD may lead to the discovery of useful biomarkers in allo-HSCT and GVHD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Herbert “Skip” Virgin and Darren Kreamalmeyer for providing IFNγ−/− and IFNγR−/− mice. They also thank Jessica Su and Johnny Wei for assisting our research as summer students and Dan Link, Camille Abboud, Mark Schroeder, and Linda Eissenberg for critical reading the paper.
J.F.D. is supported by the National Cancer Institute (R01 CA83845; and R21 grants CA110489, CA132269, CA141523 P01 CA101937, P50 CA94056). J.C. is supported by the Bryan Thomas Campbell Foundation, the Molecular Imaging Center Pilot Research Project 2010 Awards (P50 CA94056), the Translational Oncology Group (Washington University School of Medicine), the Siteman Cancer Center Research Development Awards in Developmental Therapeutics, and the American Cancer Society Institutional Research Grant (IRG-58-010-53). D.R.P.-W. is supported by P50 CA94056.
National Institutes of Health
Authorship
Contribution: J.C. and J.F.D. designed and analyzed the experiments and wrote the paper; J.C., E.D.Z., and J.R. performed the animal study; J.C., E.D.Z., L.C., J.L.P., and D.R.P.-W. carried out BLI; J.C. and E.D.Z. performed in vitro experiments including MLRs; M.L.C. and J.R. performed MLR experiments involving serial dilutions of Tregs; and all authors discussed the results and commented on the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John F. DiPersio, Division of Oncology, Department of Medicine, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8007, St Louis, MO 63110; e-mail: jdipersi@dom.wustl.edu.

![Figure 1. IFNγR−/− Tconvs do not cause life-threatening GVHD. (A) Effect of IFNγR on GVHD and survival (a pool of 4 independent experiments). Allo-HSCT (B6 [H-2b] → Balb/c]H-2d, CD45.2+]) was performed as follows. T cell–depleted bone marrow cells (TCD BMs; 5 × 106; CD45.1+ B6) and 5 × 105 Tconvs (CD45.2+ B6, either WT or IFNγR−/−) were injected into lethally irradiated (925cGy) Balb/c recipient mice. XRT: irradiation control, BM: TCD BM only, WT: TCD BM + WT Tconv, IFNγR−/−: TCD BM + IFNγR−/−Tconv, IFNγ−/−: TCD BM + IFNγ−/− Tconv. (B-D) One hundred percent donor chimerism is achieved in the IFNγR group. Peripheral blood was analyzed at day 30 after allo-HSCT. The IFNγR group shows higher CD3+ T cells and B220+ B cells in peripheral blood at day 30 after HSCT (a pool of 3 independent experiments) and better weight maintenance compared with the WT group (n = 4; one representative of 4 independent experiments). (E) Tissue sections of skin, liver, and intestine were graded by a veterinary pathologist in blinded fashion on day 20 after allo-HSCT for acute GVHD according to the Lerner grading system (see “Methods” for details).21](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/19/10.1182_blood-2012-01-403196/4/m_zh89991298600001.jpeg?Expires=1769201403&Signature=brmtoXAVvHyQrPG6cm5WfcfLa4X1NW775RwyrgXw4sK85MMuCfi-x8Iv8q4t-ivplgeg317lu3RTGa0hvquxwAvE37pNrS-bTEacBkMMLDPJf0qCpeRzpQMwVq0lW0TTkBY9TmyDg0CvTgd3pqtR4qd9yiaNAazbwH9WSbbjfL6~W3r1mUC06Vj33hs6jxheQOLNJfPkQNC2jkoEsccwiKk5~WfKYLFM6ujwqPNVP5cGsRo5mvBpPOBy1rkpwh334rgCOA3InKgmvaQp1Kw23ei6l9Z17D8pVsOpWDDxfCPbQS2PhjqmayzudTnYMuM9jRXaF6H5oABJIMGlgbkNcw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. IFNγR is required for Tregs to function appropriately in vivo. (A) Tregs also up-regulate both CXCR3 and IFNγR when activated (right panels). Mean and standard deviation of activated Tregs are as follows (n = 3). IFNγR+CXCR3−: 16.8% ± 7.0%, IFNγR-CXCR3−: 1.39% ± 1.2%, IFNγR+CXCR3+: 79.1% ± 6.1%, and IFNγR-CXCR3+: 2.7% ± 1.8%. (B) IFNγR Tregs were equally suppressive as WT Tregs in in vitro MLR assays ([3H]-thymidine incorporation was measured). Tregs were serial-diluted. The experiment was performed in triplicate or quadruplicate. Shown is 1 representative of 3 independent experiments with similar results. (C) IFNγR−/− Tregs do not suppress GVHD (B6 → Balb/c). TCD BM (B6, 5 × 106; CD45.1+) were injected into lethally irradiated Balb/c mice, followed by DLI of 2 × 106 pan T (B6, CD45.2+) and 1.5 × 106 Tregs (B6, CD45.2+). Data represent the pool of 2 independent experiments. (D) WT Tregs suppress GVHD induced by WT Tconvs but not by IFNγ−/− Tconv. Both Tregs and Tconvs (5 × 105 cells each) were injected along with TCD BM (5 × 106 cells) at day 0. Data represent the pool of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/19/10.1182_blood-2012-01-403196/4/m_zh89991298600004.jpeg?Expires=1769201403&Signature=3WAHgIBeUXSLZFv4x2Zr93recV-AI767-TQq2KJhJBTf8YbFrJPmnP7vau1nDSc8FmI6J-kaRTtV~sOZiCdteu64XUMf1FO-Tj7kAWrCzG1gyQcrNvGaoy4CppjO4G5FJNhCJX4l1FTBDrUxZseKq9KnvjnCErcMmKVpIsgTssVe3M0jdfCdtdPqxmEdmW2aZ3zENGsg7Fl8rkF-HIJHrlRryP8Gw-j8maOEtmcOqyOdzMm7n9QCwEUMsnv4ihXvsEUXCdb8Nh0ey4Cw0MWkWuYQ00CwVt3tjMmx8Ht88v3Vnp-836rayD418VjyTu2DNdfwrSMQ3TSX7pwv5yGmXA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal