Abstract
The devastating effect of ischemic stroke is attenuated in mice lacking conventional and unconventional T cells, suggesting that inflammation enhances tissue damage in cerebral ischemia. We explored the functional role of αβ and γδ T cells in a murine model of stroke and distinguished 2 different T cell–dependent proinflammatory pathways in ischemia-reperfusion injury. IFN-γ produced by CD4+ T cells induced TNF-α production in macrophages, whereas IL-17A secreted by γδ T cells led to neutrophil recruitment. The synergistic effect of TNF-α and IL-17A on astrocytes resulted in enhanced secretion of CXCL-1, a neutrophil chemoattractant. Application of an IL-17A–blocking antibody within 3 hours after stroke induction decreased infarct size and improved neurologic outcome in the murine model. In autoptic brain tissue of patients who had a stroke, we detected IL-17A–positive lymphocytes, suggesting that this aspect of the inflammatory cascade is also relevant in the human brain. We propose that selective targeting of IL-17A signaling might provide a new therapeutic option for the treatment of stroke.
Introduction
Ischemic stroke represents a major cause of disability and death in the western world.1 Although infiltration of inflammatory leukocytes is a well-described feature of human stroke,2 the perspective that activation of the immune systems is a bystander phenomenon secondary to ischemic tissue damage has changed. Currently, the activation of the immune system is recognized as a major element in all stages of the pathophysiology of stroke, including long lasting regenerative processes.1,3
Release of danger molecules, local expression of proinflammatory cytokines, the subsequent expression of endothelial adhesion molecules, and breakdown of the blood-brain barrier are among the initial events after arterial occlusion.3 These events are followed by an amplification of the postischemic inflammation that involves both resident brain cells and infiltrating immune cells. With the use of a mouse model of middle cerebral artery occlusion (MCAO), our group has previously shown a sequentially organized accumulation of immune cells of both the innate and adaptive immune systems in the ischemic brain.4 The cellular infiltrate is dominated by neutrophils, macrophages, and microglia, but also includes T, natural killer, and dendritic cells.
At this early stage, different T-cell subpopulations play important roles even if their absolute abundance in the ischemic brain is low. CD4+ and CD8+ T cells, as well as γδ T cells, promote further tissue damage,5-8 whereas regulatory T cells and B cells are protective.9,10 Cytokines involved in the proinflammatory response include IL-1β, IL-12, and IL-23, as well as interferon γ (IFN-γ), IL-17A, and TNF-α. In contrast IL-4, TGF-β, and mostly IL-10 are part of protective pathways.9,11,12 However, the specific integration of each cell type and cytokine in the postischemic inflammatory network still has to be elucidated.
In sterile inflammations, including ischemia, IL-17A can be crucial for chemokine induction.13,14 Importantly, IL-17A can be rapidly released by γδ T cells in response to cytokine activation or engagement of innate receptors, in the absence of TCR activation.15 Beside IL-17A, IFN-γ pathways are also implicated in ischemia/reperfusion (I/R) injury.13,16 In autoimmunity, IFN-γ production is associated with induction of MHCII expression, production of chemokines, and activation of macrophages.17
Our analysis of the evolving local and systemic inflammatory responses after stroke has yielded 2 new distinct and cross-linked pathways: First, IFN-γ produced by αβ T cells induces the expression of TNF-α in macrophages. Second, γδ T cells lead to neutrophil infiltration via the IL-17A and TNF-α synergistically induced expression of CXCL-1 in astrocytes. Finally, we provide evidence for the involvement of the IL-17A pathway in human stroke and suggest IL-17A as a new target for stroke therapy.
Methods
Animals
All animal experiments were approved by the local animal care committee (Behörde für Lebensmittelsicherheit und Veterinärwesen) and were conducted according to the published recommendations for research in mechanism-driven basic stroke studies.18 Transgenic mice were backcrossed at least 10 generations to the C57BL/6 background. C57BL/6 mice and mice deficient in recombination activating gene-1 (Rag1−/−) were obtained from The Jackson Laboratory. Mice devoid of γδ T cells (Tcrd−/−; The Jackson Laboratory) and Tcrd-H2BEGFP19 mice were kindly provided by I.P. (Hannover Medical School). IL-17RA knockout mice (Il17ra−/−) were obtained from Amgen.20 CXCR2 knockout mice (Cxcr2−/−; The Jackson Laboratory) were kindly provided by Ulf Panzer (University Hospital Hamburg Eppendorf). Myeloperoxidase (MPO) knockout mice (MPO_tm1lus; Mpo−/−) were kindly provided by Stephan Baldus (University Hospital Hamburg Eppendorf).
In vivo stroke model
All mice were randomized, and the scientists performing the experiments were blinded. Temporary MCAO was done as previously described.4 MCAO was achieved with the intraluminal filament method (6-0 nylon) for 1 hour. All mice (20-25 g; 12 weeks old; TVH, University Medical Center Hamburg-Eppendorf) were anesthetized (isoflurane 1%-2% vol/vol oxygen) and underwent analgesia (buprenorphine 0.03 mg/kg of body weight intraperitoneally every 12 hours for 24 hours). All mice were monitored for blood pressure, heart rate, rectal body temperature, and cerebral blood flow with the use of transcranial temporal laser Doppler scans. After stroke induction, every mouse was repeatedly scored on a scale from 0 to 5 (0, no deficit; 1, preferential turning; 2, circling; 3, longitudinal rolling; 4, no movement; and 5, death) immediately after reawakening and every day until killing. The cerebral blood flow in the area of the MCA showed a reduction by ∼ 90%, which did not differ between groups (data not shown). Blood pressure, heart rate, and rectal body temperature also showed no difference in our cohorts. Mice were killed 1, 3, or 7 days after reperfusion with the use of isoflurane and decapitation. Only mice with a score more than or equal to 1 after reawakening were included for stroke size analysis, and only animals with a visible cortical infarct were included for FACS analysis of infiltrating cells. For analysis of infarct size the detailed experimental description can be found in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Antibody treatment
Animals were treated with 500 μg of mouse monoclonal anti–murine IL-17A antibody21 (Clone MM17F3; 16.6 mg/kg of bodyweight) or with 500 μg of isotype-control antibody (IgG1). Mice were injected intraperitoneally once at 3 hours, 6 hours, or 12 hours after onset of ischemia. Polyclonal goat anti–murine CXCR2 serum was a kind gift of T. J. Standiford (University of Michigan Health System). The serum was produced by immunization of a goat with murine rt CXCR2 peptide. The peptide sequence, Met-Gly-Glu-Phe-Lys-Val-Asp-Lys-Phe-Asn-Ile-Glu-Asp-Phe-Phe-Ser-Gly, is a portion of the 7-transmembrane receptor that resides on the cell surface and has previously been shown to be the binding site for ligands.22 In CXCR2 neutralization experiments, 0.5 mL of goat/anti–murine CXCR2 serum or control goat serum was administered intraperitoneally 2 hours before 1 hour MCAO, followed by 0.25 mL 48 hours after stroke.
Cell transfer
T cells were purified from total splenocytes from wild-type (wt), Tcrd−/−, or Il17ra−/− mice by negative selection with the use of magnetic beats (Miltenyi Biotec). To obtain exclusively αβ T cells, T cells were prepared from splenocytes from Tcrd−/− mice. The cell purity was greater than 99%. In adoptive transfer experiments, Rag1−/− mice were injected intravenously with 1 × 107 T cells from C57BL/6 or Tcrd−/− mice 60 minutes before onset of ischemia.
Antibodies and flow cytometry
Flow cytometry for the analysis of cell types was performed as previously described.4 T cells were stimulated with phorbol 12-myristate 13-acetate (100 ng/mL; Sigma-Aldrich) and ionomycin (1 μg/mL; Sigma-Aldrich) and in the presence of brefeldin A (3 μg/mL; eBioscience) for 4 hours. For intracellular staining of monocytes, neutrophils, and microglia, intracellular transport was blocked with brefeldin A (3 μg/mL; eBioscience) for 3 hours. The detailed experimental description can be found in supplemental Methods.
Mixed cell culture
Primary cultures of mixed glial cells were prepared from 1- to 2-day-old mice. The detailed experimental description can be found in supplemental Methods.
ELISA
CCL-2 and CXCL-1 protein levels were determined in cell culture supernatants by ELISA according to the manufacturer (BD Bioscience and R&D Systems, respectively).
RNA isolation and quantitative real-time PCR
Real-time PCR primers were obtained from Applied Biosystems (Actinb Mm00607939_s1; CXCL-1 Mm00433859_m1; CCL-2 Mm00441242_m1; CXCL-2 Mm00436450_m1; CCL-20 Mm00444228_m1; MMP3 Mm00440295_m1; MMP13 Mm00439491_m1), and probe mixtures were purchased from Fermentas. The detailed experimental description can be found in supplemental Methods.
Immunohistochemistry
For histologic analysis of mouse brains, animals were perfused with 4% buffered formalin. Brains were stained according to standard immunohistochemistry procedures with antibodies against GFAP (1:200; Dako), Iba-1 (1:200; Wako), and Ly6G clone 1A8 (1:1000; Biolegend). For analysis of autoptic human brain tissue, cases were selected from the files of the Institute of Neuropathology at the University Medical Center Hamburg-Eppendorf. Brain specimens had been fixed in 4% buffered formalin for at least 3 weeks before paraffin-embedding. Brain sections (3 μm thick) were stained according to standard immunohistochemistry procedures with antibodies against MPO (1:2500; Dako), CD3 (clone SP7; 1:200; Thermo Scientific), IL-17A (affinity-purified polyclonal goat IgG; AF-317; 1:50; R&D Systems), TCR γ chain (M1 γ 3.20; 1:50; Thermo Scientific), CXCL-1 (MAB275; 1:20; R&D Systems), CXCL-8 (MAB208; 1:20; R&D Systems), TNF-α (ab1793; 1:20; Abcam), and IFN-γ (ab9657; 1:100; Abcam). Immunofluorescence double-staining was performed by simultaneous incubation with goat/anti–human IL-17A antibody (1:10; R&D Systems) and rabbit/anti–human CD3 monoclonal antibody (Clone SP7; 1:20; Thermo Scientific). The detailed experimental description can be found in supplemental Methods.
Statistics
Data are reported as mean ± SD. Statistical analyses were performed with the appropriate test indicated in the figure legends. Briefly, the Student t test was used to compare infarct volumes; Mann-Whitney U test for the comparison of clinical scores, and 1-way ANOVA for multiple comparisons with Bonferroni posthoc test, after validating the normal distribution of these datasets (Kolmogorov-Smirnov test). P values < .05 were considered statistically significant.
Results
Infiltrating conventional and unconventional T cells exhibit distinct cytokine expression patterns after migration into the ischemic hemisphere
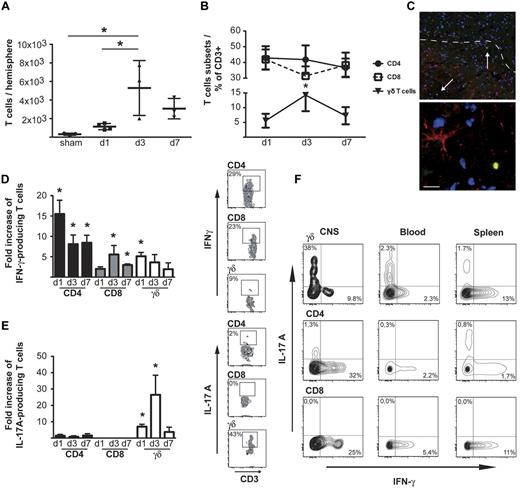
The healthy brain is only patrolled by few T cells, but this situation changes dramatically after I/R injury. Already 24 hours after reperfusion, T-cell numbers in the infarcted hemisphere rose from a basal mean ± SD of 313 ± 113 in sham-operated mice to 1114 ± 283 per hemisphere, reaching a maximum of 5293 ± 2958 cells on day 3 after ischemia (Figure 1A). At day 7, numbers of T cells decreased again. Analysis of the frequency of T-cell subsets found that CD4+ and CD8+ T cells, although more abundant in absolute numbers, remained stable over time (32%-42% of total CD3+ cells), whereas γδ T cells increased both in absolute numbers and in frequency on day 3 (from < 6% to a maximum of 14% ± 1.5%; P < .05; Figure 1B). Importantly, these γδ T cells were found in the penumbra of the stroke as visualized by green fluorescent protein (GFP) in Tcrd-H2BEGFP mice (Figure 1C), suggesting site-specific migration properties.
Conventional and unconventional T cells infiltrate the ischemic hemisphere and produce high amounts of cytokines at the site of the injury. (A) Absolute numbers of T lymphocytes in ischemic hemispheres at days 1, 3, and 7 after MCAO. Cell counts were determined by flow cytometric analysis of CNS-infiltrating cells after Percoll density centrifugation and staining for CD45 and CD3. For absolute quantification, TrueCount tubes were used. (B) Frequency of CD4+, CD8+, and γδ T lymphocytes in ischemic hemispheres at days 1, 3, and 7 after stroke. Data were obtained after flow cytometric analysis of CNS-infiltrating cells stained for CD45, CD3, NK1.1, CD4, CD8, and γδ T-cell receptor. (C) GFP-positive γδ T cells were visualized in the penumbra area of the ischemic hemisphere in Tcrd-H2BEGFP mice 3 days after MCAO (red indicates GFAP-positive astrocytes; blue, DAPI nuclear staining; scale bar = 20 μm). Flow cytometric analysis of IFN-γ (D) and IL-17A (E) produced by CD4+, CD8+ and γδ T cells isolated from ischemic hemispheres at different days after stroke induction. Right panels show intracellular stainings for IFN-γ and IL-17A of gated CD4+, CD8+, and γδ T cells of 1 representative experiment at day 3. (F) Comparison of IFN-γ and IL-17A expression by T cells isolated from brain, peripheral blood, and spleen 3 days after stroke. (A,B,D,F) The graphs show mean ± SD of 12-14 animals per group, in 3-4 independent experiments for each time point. Statistical significances analyzed by 1-way ANOVA with Bonferroni posthoc test (*P < .05) in all cases.
Conventional and unconventional T cells infiltrate the ischemic hemisphere and produce high amounts of cytokines at the site of the injury. (A) Absolute numbers of T lymphocytes in ischemic hemispheres at days 1, 3, and 7 after MCAO. Cell counts were determined by flow cytometric analysis of CNS-infiltrating cells after Percoll density centrifugation and staining for CD45 and CD3. For absolute quantification, TrueCount tubes were used. (B) Frequency of CD4+, CD8+, and γδ T lymphocytes in ischemic hemispheres at days 1, 3, and 7 after stroke. Data were obtained after flow cytometric analysis of CNS-infiltrating cells stained for CD45, CD3, NK1.1, CD4, CD8, and γδ T-cell receptor. (C) GFP-positive γδ T cells were visualized in the penumbra area of the ischemic hemisphere in Tcrd-H2BEGFP mice 3 days after MCAO (red indicates GFAP-positive astrocytes; blue, DAPI nuclear staining; scale bar = 20 μm). Flow cytometric analysis of IFN-γ (D) and IL-17A (E) produced by CD4+, CD8+ and γδ T cells isolated from ischemic hemispheres at different days after stroke induction. Right panels show intracellular stainings for IFN-γ and IL-17A of gated CD4+, CD8+, and γδ T cells of 1 representative experiment at day 3. (F) Comparison of IFN-γ and IL-17A expression by T cells isolated from brain, peripheral blood, and spleen 3 days after stroke. (A,B,D,F) The graphs show mean ± SD of 12-14 animals per group, in 3-4 independent experiments for each time point. Statistical significances analyzed by 1-way ANOVA with Bonferroni posthoc test (*P < .05) in all cases.
To assess the potential relevance of T cells in stroke, we measured the proinflammatory cytokines they produced in the injured tissue in comparison with cytokines produced by circulating T cells. IFN-γ, mostly produced by CD4+ T cells, was increased in the brain already on the first day after stroke (Figure 1D,F). CD8+ T cells and γδ T cells showed only moderate changes in the production of IFN-γ. Remarkably, γδ T cells were the main cell population that secreted IL-17A in the ischemic brain. IL-17A–positive γδ T cells were detectable in the affected hemisphere already 6 and 12 hours after stroke (supplemental Figure 1). One day after MCAO, we observed a 7-fold increase of IL-17A in γδ T cells and a more than 25-fold increase on day 3 (Figure 1E-F). The production of TNF-α by all 3 T-lymphocyte subsets was only marginally increased (2- to 3-fold) in the ischemic hemisphere compared with corresponding T cells from the blood (supplemental Figure 1). In summary, T cells infiltrated the ischemic brain in the first hours after reperfusion, and the number of infiltrating T cells reached maximum levels 3 days after stroke. These T cells displayed a distinct pattern of cytokine production, although conventional T cells were the main source of IFN-γ, γδ T cells produced IL-17A.
T cell–derived IFN-γ induces TNF-α production by infiltrating macrophages
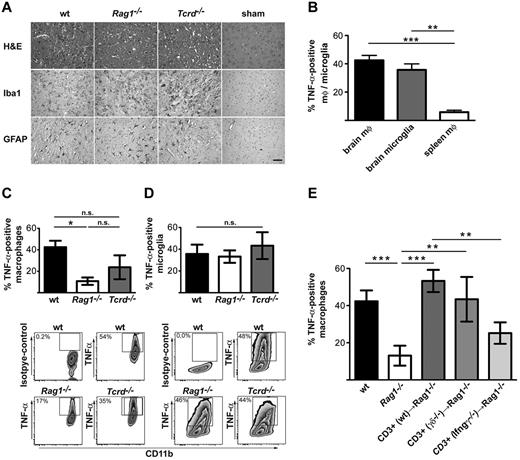
Possible target cells of T cell–derived cytokines include macrophages and microglia. We have previously shown that macrophages and microglia are rapidly recruited to the injured brain after stroke.4 This infiltration was independent of the presence of T cells, because it occurred at similar levels in T cell–deficient (Rag1−/−) animals and in animals that lack γδ T cells (Figure 2A). However, although in wt mice the brain-infiltrating macrophages produce high amounts of TNF-α compared with spleen macrophages (Figure 2B), the production of TNF-α is markedly reduced in animals that lack T cells. This defect can mostly be attributed to the lack of conventional αβ T cells, because γδ T cell–deficient animals show a less-prominent decrease in the TNF-α production of macrophages (Figure 2C). In contrast to macrophages, microglial cells produced similar amounts of TNF-α, irrespective of the presence of T cells (Figure 2D). TNF-α production by spleen macrophages was not affected by the presence or absence of T cells in this model (supplemental Figure 2). To further verify the role of specific T-cell subsets on the induction of TNF-α production by CNS-infiltrating macrophages, we reconstituted Rag1−/− mice with different T-cell types (Figure 2E). Transfer of 1 × 107 unfractionated T cells from wt mice 1 hour before MCAO rendered the TNF-α response in brain macrophages equal to that in wt mice. If the T cells were isolated from Ifng−/− mice, TNF-α production by macrophages could not be reconstituted, indicating that this cytokine is required for the activation of the macrophages. Interestingly, when T cells were isolated from Tcrd−/− animals (and thus were pure conventional T-αβ cells), the TNF-α could be fully reconstituted, indicating that γδ T cells are not critical for macrophage activation. Altogether, our data suggest that IFN-γ produced by conventional αβ T cells is required to induce TNF-α production in the brain-infiltrating macrophages after stroke induction.
Induction of TNF-α in macrophages by αβ T cell–derived by IFN-γ. (A) Immunohistochemical staining of macrophages/microglia (Iba1) and astrocytes (GFAP) in wt, Rag1−/−, Tcrd−/−, and sham-operated wild type wt mice after MCAO (scale bar = 50 μm). (B) Frequency of TNF-α–positive brain macrophages, microglia, and peripheral (spleen) macrophages analyzed 3 days after MCAO. Brain macrophages were identified as CD45high, CD11b+, or CD11c− and were distinguished from microglia by the higher expression of CD45. Frequency of TNF-α–positive brain macrophages (C) and microglia (D) in wt, Rag1−/−, and Tcrd−/− mice analyzed by flow cytometry 3 days after stroke. Representative dot plots show CD11b+ CD45high and CD11b+ CD45intermediate-gated populations, identifying macrophages and microglia, respectively. (E) In this experiment, Rag1−/− mice were reconstituted 1 hour before stroke induction with 1 × 107 CD3+ cells isolated from wt, Tcrd−/−, or Ifng−/− mice. The production of TNF-α by macrophages was analyzed 3 days after stroke by flow cytometry. In all experiments, data show the means ± SDs of 9-12 animals per group, analyzed in 3-4 independent experiments, and the statistical analysis with the use of 1-way ANOVA with Bonferroni posthoc test. **P < .01, ***P < .001.
Induction of TNF-α in macrophages by αβ T cell–derived by IFN-γ. (A) Immunohistochemical staining of macrophages/microglia (Iba1) and astrocytes (GFAP) in wt, Rag1−/−, Tcrd−/−, and sham-operated wild type wt mice after MCAO (scale bar = 50 μm). (B) Frequency of TNF-α–positive brain macrophages, microglia, and peripheral (spleen) macrophages analyzed 3 days after MCAO. Brain macrophages were identified as CD45high, CD11b+, or CD11c− and were distinguished from microglia by the higher expression of CD45. Frequency of TNF-α–positive brain macrophages (C) and microglia (D) in wt, Rag1−/−, and Tcrd−/− mice analyzed by flow cytometry 3 days after stroke. Representative dot plots show CD11b+ CD45high and CD11b+ CD45intermediate-gated populations, identifying macrophages and microglia, respectively. (E) In this experiment, Rag1−/− mice were reconstituted 1 hour before stroke induction with 1 × 107 CD3+ cells isolated from wt, Tcrd−/−, or Ifng−/− mice. The production of TNF-α by macrophages was analyzed 3 days after stroke by flow cytometry. In all experiments, data show the means ± SDs of 9-12 animals per group, analyzed in 3-4 independent experiments, and the statistical analysis with the use of 1-way ANOVA with Bonferroni posthoc test. **P < .01, ***P < .001.
IL-17A produced by γδ T cells attracts neutrophils to the infarcted hemisphere
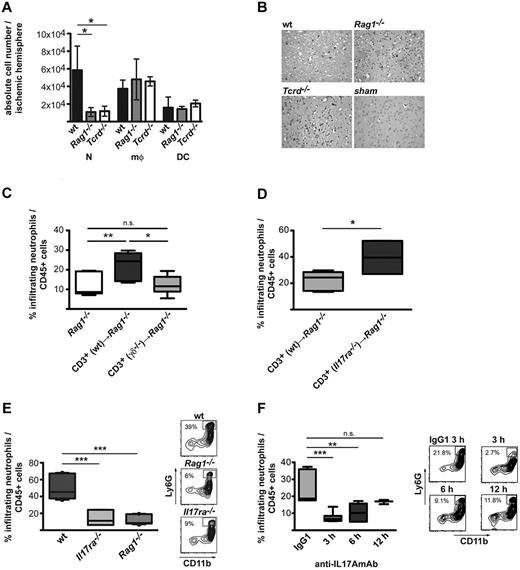
While analyzing the brain-infiltrating cells in the different T cell–deficient animals, a major difference in the composition of the infiltrate became apparent. Both Rag1−/− and Tcrd−/− animals showed dramatically reduced numbers of neutrophils in the ischemic hemisphere 3 days after stroke induction compared with wt mice (1.1 × 104 ± 0.5 × 104 and 1.2 × 104 ± 0.5 × 104, respectively, compared with 6 × 104 ± 2.7 × 104 in the wt mice; Figure 3A-B). As shown in the previous paragraph, the numbers of macrophages and microglial cells were similar (Figure 3A; supplemental Figure 2). This marked decrease in neutrophils in the ischemic brain could only be reversed when the lymphocyte compartment of the T cell–deficient mice was reconstituted before stroke induction with cells that included the γδ T-cell subpopulation, because reconstitution with unfractionated T cells from the Tcrd−/− animals were unable to attract further neutrophils to the brain (Figure 3C).
IL-17A secreted by γδ T cells after MCAO attracts neutrophils to the injured site. (A) Absolute numbers of neutrophils (N; Ly6G+, CD11b+), macrophages (mφ; Ly6G−, CD11b+), and dendritic cells (DC; CD11c+, CD11b+) in ischemic hemispheres of wt, Rag1−/−, and Tcrd−/− mice 3 days after MCAO. Cell counts were determined by flow cytometric analysis of CNS-infiltrating cells with the use of TrueCount tubes. The graph shows mean ± SD of 9-12 animals per group analyzed in 4 independent experiments. (B) Immunohistochemical staining of neutrophils (Ly6G) in wt, Rag1−/−, Tcrd−/−, and sham-operated wt mice 3 days after MCAO (scale bar = 50 μm). (C) Percentage of neutrophils in the CNS-infiltrating cells of Rag1−/− mice reconstituted 1 hour before stroke induction with 1 × 107 CD3+ cells isolated from wt or Tcrd−/− mice. (D) Percentage of neutrophils in the CNS-infiltrating cells of Rag1−/− mice reconstituted 1 hour before stroke induction with 1 × 107 CD3+ cells isolated from wt or Il17ra−/− mice. The graph shows mean ± SD of 6-8 animals per group analyzed in 3 independent experiments. (E) Frequency of neutrophils eluted from the ischemic hemispheres of Rag1−/− and Il17ra−/− mice in comparison with wt animals. A representative dot plot displaying gated CD45+ cells is shown in the right panel. (F) Percentages of neutrophils in the infiltrating cells of wt animals after administration of 500 μg of anti-IL17A antibodies (MM17F3) 3, 6, and 12 hours or of 500 μg of isotype control antibodies 3 hours after MCAO treatment. Representative plots are shown for isotype control (mouse IgG1) and anti–IL-17A treatment at the indicated time points. The graphs show in all cases the means ± SDs of 9-12 animals per group analyzed 3 days after MCAO in 3 or 4 independent experiments. (A,C,E,F) One-way ANOVA with Bonferroni posthoc test was used to assess statistical significance. **P < .01, ***P < .001. (D) Student t test with *P < .05.
IL-17A secreted by γδ T cells after MCAO attracts neutrophils to the injured site. (A) Absolute numbers of neutrophils (N; Ly6G+, CD11b+), macrophages (mφ; Ly6G−, CD11b+), and dendritic cells (DC; CD11c+, CD11b+) in ischemic hemispheres of wt, Rag1−/−, and Tcrd−/− mice 3 days after MCAO. Cell counts were determined by flow cytometric analysis of CNS-infiltrating cells with the use of TrueCount tubes. The graph shows mean ± SD of 9-12 animals per group analyzed in 4 independent experiments. (B) Immunohistochemical staining of neutrophils (Ly6G) in wt, Rag1−/−, Tcrd−/−, and sham-operated wt mice 3 days after MCAO (scale bar = 50 μm). (C) Percentage of neutrophils in the CNS-infiltrating cells of Rag1−/− mice reconstituted 1 hour before stroke induction with 1 × 107 CD3+ cells isolated from wt or Tcrd−/− mice. (D) Percentage of neutrophils in the CNS-infiltrating cells of Rag1−/− mice reconstituted 1 hour before stroke induction with 1 × 107 CD3+ cells isolated from wt or Il17ra−/− mice. The graph shows mean ± SD of 6-8 animals per group analyzed in 3 independent experiments. (E) Frequency of neutrophils eluted from the ischemic hemispheres of Rag1−/− and Il17ra−/− mice in comparison with wt animals. A representative dot plot displaying gated CD45+ cells is shown in the right panel. (F) Percentages of neutrophils in the infiltrating cells of wt animals after administration of 500 μg of anti-IL17A antibodies (MM17F3) 3, 6, and 12 hours or of 500 μg of isotype control antibodies 3 hours after MCAO treatment. Representative plots are shown for isotype control (mouse IgG1) and anti–IL-17A treatment at the indicated time points. The graphs show in all cases the means ± SDs of 9-12 animals per group analyzed 3 days after MCAO in 3 or 4 independent experiments. (A,C,E,F) One-way ANOVA with Bonferroni posthoc test was used to assess statistical significance. **P < .01, ***P < .001. (D) Student t test with *P < .05.
We and others have previously shown that γδ T cells produce IL-17A in the context of stroke and that IL-17A is known to induce the expression of proinflammatory mediators by stromal cells, which in turn leads to the recruitment and activation of neutrophils.23 We thus hypothesized that the lack of IL-17A signaling would be relevant for the attraction of neutrophils to the ischemic brain. Indeed, animals deficient in the subunit A of the IL-17–receptor heterodimer, Il17ra−/− mice, showed a marked reduction of invading neutrophils after stroke, similar to Rag1−/− mice and to mice deficient in γδ T cells (Figure 3E; supplemental Figure 3). The role of IL-17A derived from the T-cell compartment could be further confirmed in transfer experiments with T cells from Il17ra−/− mice. Because of a defect inhibitory feedback loop, the frequency of IL-17A–producing T cells as well as their IL-17A expression and secretion are increased in Il17ra−/− mice.24 Congruently, Rag1−/− mice reconstituted with unfractionated T cells from Il17ra−/− mice showed a further significant increase of invading neutrophils compared with Rag1−/− mice reconstituted with T cells from wt mice (Figure 3D).
Because IL-17RA is the receptor not only for IL-17A and IL-17F but also for IL-17E23 and IL-17C,25 we sought to directly block IL-17A binding to the receptor with specific anti–IL-17A antibodies.21 When anti–IL-17A antibodies were given intravenously as a single application at different time points after stroke, we could see that the neutrophil infiltration was greatly reduced 24 hours and 72 hours after stroke if the antibody was administered at the latest 3, respectively, 6 hours after stroke induction, which is a clinically relevant time window but had no effect if given 12 hours after stroke (Figure 3F; supplemental Figure 3). The reduced neutrophil infiltration in Il17ra−/− mice and in mice treated with neutralizing antibodies was not because of reduced neutrophil levels in the blood, because relative neutrophil counts 3 days after stroke did not differ between groups (supplemental Figure 3).
Recruitment of neutrophils to the infarct site depends on CXCL-1 induced on IL-17 signaling
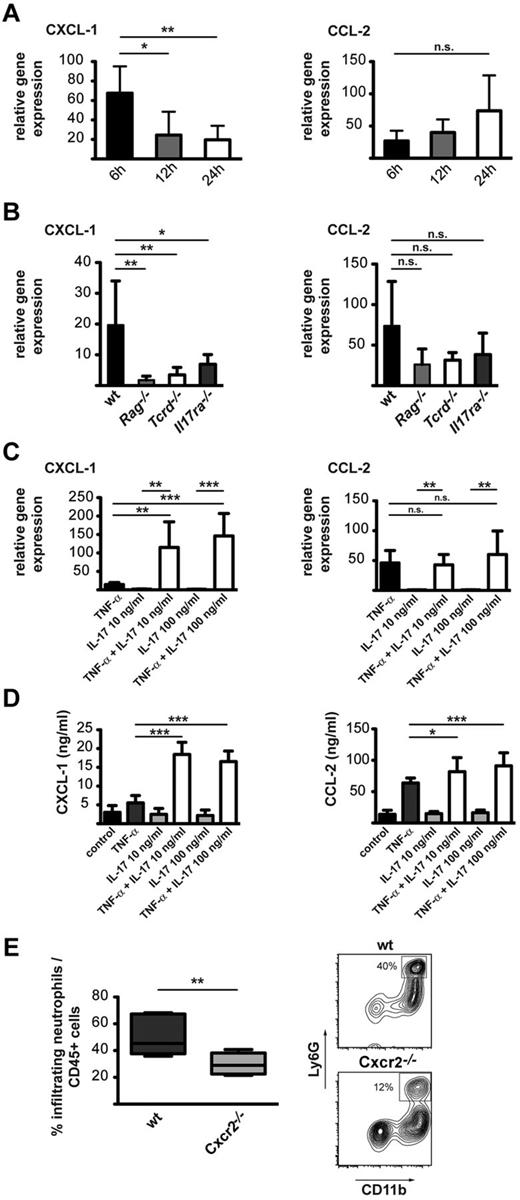
Neutrophils are not direct targets of IL-17A, because they do not express the IL-17RC subunit,23 but they are known to readily react to chemoattractants released on interaction of IL-17A with epithelial, endothelial, and other stromal cells. We, therefore, hypothesized that lL-17A interacts with local brain cells such as astrocytes or microglia and induces production of neutrophil-attracting chemokines. The first approach was to assess if the synthesis of neutrophil chemoattractants, which are rapidly up-regulated after stroke in the ischemic hemisphere (Figure 4A; supplemental Figure 4), was impaired in our models of decreased neutrophil infiltration. Indeed, Rag1−/−, Tcrd−/−, and Il17ra−/− mice showed reduced levels of CXCL-1, a chemokine that mainly recruits neutrophils, whereas the expression levels of CCL-2, CXCL-2, and CCL-20 were not significantly affected (Figure 4B; supplemental Figure 4).
TNF-α and IL-17A act synergistically to induce CXCL-1 expression in astrocytes. (A) Relative gene expression of CXCL-1 and CCL2 in the stroked brain. RNA was obtained from the affected hemisphere 6, 12, and 24 hours after MCAO (n = 6 mice). (B) Relative gene expression of CXCL-1 and CCL2 in the stroked brain of wt, Rag1−/−, Tcrd−/−, and Il17ra−/− mice. RNA was obtained from the affected hemisphere 24 hours after MCAO (n = 6 mice). (C) Changes in CXCL-1 and CCL-2 RNA expression in primary astrocytes 24 hours after stimulation with TNF-α, IL-17A, or both. (D) Quantification of CCL-2 and CXCL-1 secreted by primary astrocytes 24 hours after cytokine stimulation by ELISA. Graphs show means ± SDs of 5 independent experiments and the statistical significance. (E) Frequency of neutrophils eluted from the ischemic hemispheres of Cxcr2−/− mice in comparison with wt mice 3 days after MCAO. The graphs show the means ± SDs of 9-12 animals per group analyzed 3 days after MCAO in 3 or 4 independent experiments. A representative dot plot displaying gated CD45+ cells is shown in the right panel. (A-D) One-way ANOVA with Bonferroni posthoc test. *P < .05, **P < .01, ***P < .001. (E) Student t test with **P < .01.
TNF-α and IL-17A act synergistically to induce CXCL-1 expression in astrocytes. (A) Relative gene expression of CXCL-1 and CCL2 in the stroked brain. RNA was obtained from the affected hemisphere 6, 12, and 24 hours after MCAO (n = 6 mice). (B) Relative gene expression of CXCL-1 and CCL2 in the stroked brain of wt, Rag1−/−, Tcrd−/−, and Il17ra−/− mice. RNA was obtained from the affected hemisphere 24 hours after MCAO (n = 6 mice). (C) Changes in CXCL-1 and CCL-2 RNA expression in primary astrocytes 24 hours after stimulation with TNF-α, IL-17A, or both. (D) Quantification of CCL-2 and CXCL-1 secreted by primary astrocytes 24 hours after cytokine stimulation by ELISA. Graphs show means ± SDs of 5 independent experiments and the statistical significance. (E) Frequency of neutrophils eluted from the ischemic hemispheres of Cxcr2−/− mice in comparison with wt mice 3 days after MCAO. The graphs show the means ± SDs of 9-12 animals per group analyzed 3 days after MCAO in 3 or 4 independent experiments. A representative dot plot displaying gated CD45+ cells is shown in the right panel. (A-D) One-way ANOVA with Bonferroni posthoc test. *P < .05, **P < .01, ***P < .001. (E) Student t test with **P < .01.
To gain insight into cell types that responded to IL-17A and to verify the role of CXCL-1, we stimulated primary astrocyte and microglial cultures with IL-17A in the presence and absence of TNF-α. When given independently, both cytokines constituted a weak stimulus for CXCL-1 mRNA expression (Figure 4C). However, a profound synergistic effect on the expression of CXCL-1 was evident when TNF-α and IL-17A were added simultaneously to the astrocyte but not to microglia cultures (Figure 4C; supplemental Figures 5-6). Adding IL-17A did not have any effect on the expression of CCL-2, CXCL-2, and CCL-20 in astrocytes (Figure 4; supplemental Figure 4). Finally, we measured CXCL-1 and CCL-2 secreted into the supernatant of the cell cultures after TNF-α and IL-17A stimulation, and we observed that the changes in gene expression resulted in substantial differences at the protein level as well (Figure 4D). It has been proposed that IL-17A has a stabilizing effect on mRNA.26 To assess if this mechanism could also be responsible for the increased abundance of CXCL-1, we used actinomycin D to block transcription and prepared mRNA at different time points. Forty-five minutes after adding actinomycin D, CXCL-1 mRNA levels were significantly elevated in the cells that had been treated with IL-17A and TNF-α compared with TNF-α alone, and no effect was observed on CCL-2 (supplemental Figure 6).
CXCL-1 binds to its receptor CXCR-2, resulting in the recruitment of neutrophils.27 To explore the direct involvement of the CXCL-1/CXCR-2 axis in stroke, we analyzed the neutrophil infiltration in ischemic hemispheres of Cxcr2−/− mice. Three days after stroke, Cxcr2−/− mice exhibited a significant decrease in infiltrated neutrophils compared with wt animals (Figure 4E).
To assess the importance of the CXCL-1/CXCR-2 axis in vivo, we analyzed effects of neutralizing polyclonal goat/anti–mouse CXCR-2 serum on stroke size. Administration of neutralizing CXCR-2 serum 2 hours before stroke induction resulted in significantly smaller infarct sizes 3 days after MCAO (supplemental Figure 7).
Taken together, our data show that astrocytes respond to IL-17A together with TNF-α in the ischemic environment by producing CXCL-1, which in turn attracts neutrophils to the infarcted site, thus amplifying the inflammatory response.
Role of neutrophils and neutrophil-independent IL-17A effects
In the absence of a reliable model for neutrophil depletion, we used MPO-deficient (Mpo−/−) mice. MPO is an abundant heme protein in neutrophils, with potent vascular inflammatory properties. Mpo−/− animals had significantly smaller infarcts and improved neurologic outcome after stroke (supplemental Figure 8).
IL-17A has well-described neutrophil-independent effects.23 By analyzing potential candidate genes, including MMP3 and MMP13, we identified MMP3 to be regulated by IL-17A in stroke. In Il17ra−/− mice MMP3 levels were significantly reduced 24 hours after stroke. In primary astrocyte cultures TNF-α and IL-17A could synergistically induce MMP3 (supplemental Figure 7). Other candidate genes such as MMP13 (supplemental Figure 7) were not altered in vivo and in vitro.
In conclusion, we show that neutrophils contribute to the tissue damage and are, therefore, one key mechanism of the IL-17A effect. However, other inflammatory mediators such as MMP3 are also regulated by IL-17A and could contribute to the further breakdown of the blood-brain barrier.28
Neutralization of IL-17A is protective in stroke
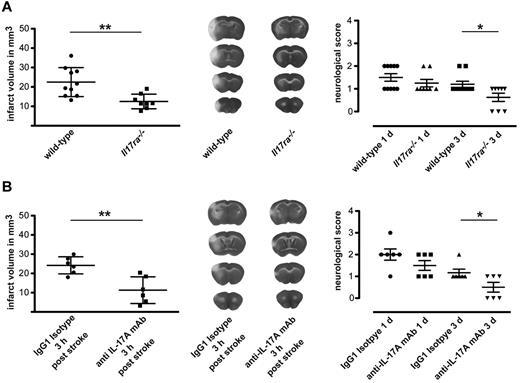
We have shown that neutrophil infiltration is markedly reduced when IL-17A engagement to its receptor is impaired. To assess if it also has a direct effect on the recovery of the animals, we analyzed infarct size and neurologic scores after MCAO. Three days after the ischemic attack, Il17ra−/− mice showed a significantly reduced infarct size and milder disability compared with wt animals (Figure 5A). In addition, and much more interesting for therapy, administration of a single dose of anti–IL-17A (500 μg) 3 hours after stroke induction also resulted in a significant reduction in the infarct size and improvement in clinical outcome compared with the injection of an isotype control antibody (Figure 5B).
Disruption of IL-17A signaling is protective in stroke. (A) Triphenyltetrazolium chloride (TTC) staining for evaluation of infarct volume at day 3 (left) and neurologic scores at days 1 and 3 (right) of wt and Il17ra−/− mice after MCAO. Data are represented as means ± SDs of 10 wt and 8 Il17ra−/− animals. (B) TTC staining evaluation of infarct volume at day 3 (left) and neurologic scores at days 1 and 3 (right) of wt animals that were treated intraperitoneally with 500 μg of anti–IL-17A (clone MM17F3) or isotype control antibodies 3 hours after onset of MCAO. Data show the means ± SDs of 6 animals per group. *P < .05, **P < .01 for infarct sizes (t test) and *P < .05 for neurologic scores (Mann-Whitney U test). Representative TTC-stained serial coronal 1-mm thick brain sections 3 days after MCAO to analyze infarct volume in wt mice, Il17ra−/− mice, wt treated with isotype control antibody, and wt mice treated with anti–IL-17A antibody.
Disruption of IL-17A signaling is protective in stroke. (A) Triphenyltetrazolium chloride (TTC) staining for evaluation of infarct volume at day 3 (left) and neurologic scores at days 1 and 3 (right) of wt and Il17ra−/− mice after MCAO. Data are represented as means ± SDs of 10 wt and 8 Il17ra−/− animals. (B) TTC staining evaluation of infarct volume at day 3 (left) and neurologic scores at days 1 and 3 (right) of wt animals that were treated intraperitoneally with 500 μg of anti–IL-17A (clone MM17F3) or isotype control antibodies 3 hours after onset of MCAO. Data show the means ± SDs of 6 animals per group. *P < .05, **P < .01 for infarct sizes (t test) and *P < .05 for neurologic scores (Mann-Whitney U test). Representative TTC-stained serial coronal 1-mm thick brain sections 3 days after MCAO to analyze infarct volume in wt mice, Il17ra−/− mice, wt treated with isotype control antibody, and wt mice treated with anti–IL-17A antibody.
IL-17A expression in lymphocytes in human stroke specimens
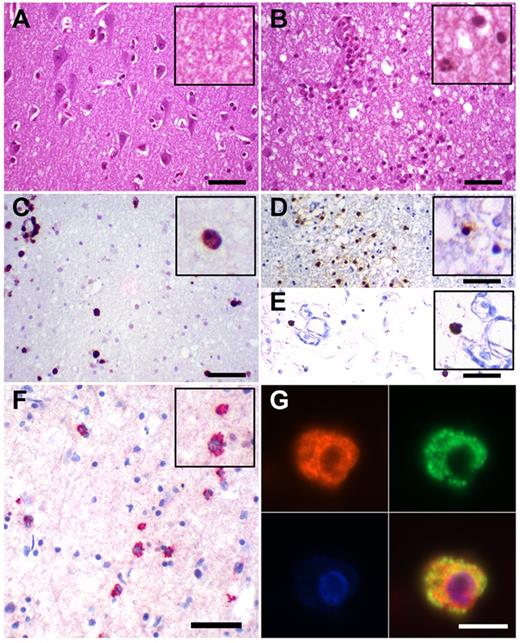
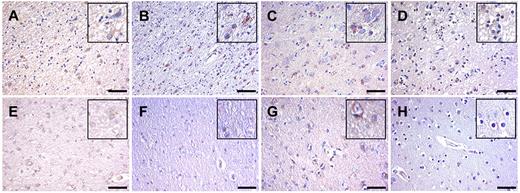
The translation of findings from the mouse model to human disease is difficult. However, given the possibility of using analogous treatment for stroke, we assessed if neutrophils and the mechanism proposed could also be relevant for humans. For this, we examined postmortem stroke tissue from patients who had died shortly after having a stroke (< 24 hours). In this tissue, H&E stainings showed cells with unequivocal physical characteristics of neutrophils (polymorphonuclear) or lymphocytes (Figure 6A-B), whereas no inflammatory cells were seen in normal brain tissue (Figure 6A; supplemental Figure 9). Immunohistochemically large numbers of MPO-positive neutrophils, fewer CD3+ T cells, and γδ T cells were identified in stroke areas (Figure 6A-D). Importantly, staining for IL-17A showed positive cells in the infarcted area (Figure 6F), which, by double immunofluorescence staining with the use of anti-CD3 antibodies, could be identified as T cells (Figure 6G), thus suggesting that the T cell/IL-17/neutrophil axis might also be involved in the pathogenesis of I/R injury after stroke in humans. Further immunohistochemical analysis found CXCL-1–, CXCL-8–, TNF-α–, and, infrequently, IFN-γ–expressing cells in stroke areas (Figure 7A-H).
IL-17A is expressed by CD3+ T cells in human brain tissue shortly after stroke. H&E staining of unaffected brain tissue (A) and ischemic brain tissue 24 hours after stroke (B). The ischemic tissue was further analyzed by immunohistochemistry for the presence of neutrophils (MPO; C); T lymphocytes (CD3; D); γδ T cells (E), and IL-17A (F). (G) Double immunofluorescence analysis of CD3 (red) and IL-17A (green) at higher magnification. Scale bars = 50 μm (A-F) and 10 μm (G).
IL-17A is expressed by CD3+ T cells in human brain tissue shortly after stroke. H&E staining of unaffected brain tissue (A) and ischemic brain tissue 24 hours after stroke (B). The ischemic tissue was further analyzed by immunohistochemistry for the presence of neutrophils (MPO; C); T lymphocytes (CD3; D); γδ T cells (E), and IL-17A (F). (G) Double immunofluorescence analysis of CD3 (red) and IL-17A (green) at higher magnification. Scale bars = 50 μm (A-F) and 10 μm (G).
Inflammatory cytokines are expressed in human stroke. Immunohistochemical analysis of cytokine expression in ischemic brain tissue (A-D) and unaffected brain tissue (E-H). Immunohistochemical analysis was performed to detect CXCL-1 (A,E), CXCL-8 (B,F), TNF-α (C,G), and IFN-γ (D,H). Scale bars = 50 μm.
Inflammatory cytokines are expressed in human stroke. Immunohistochemical analysis of cytokine expression in ischemic brain tissue (A-D) and unaffected brain tissue (E-H). Immunohistochemical analysis was performed to detect CXCL-1 (A,E), CXCL-8 (B,F), TNF-α (C,G), and IFN-γ (D,H). Scale bars = 50 μm.
Discussion
Through this study, we can now integrate the concomitant pathways of CD4+ T cells and γδ T cells in stroke pathophysiology. After stroke, IFN-γ secreted by CD4+ T cells leads to the activation of invading macrophages, whereas unconventional γδ T cells secrete IL-17A and attract neutrophils to the site of injury. Further, we describe a new role of astrocyte-derived CXCL-1 as a key mediator of the IL-17A–initiated neutrophil chemotaxis in stroke. Blockade of the IL-17A pathway within clinical relevant time points after stroke resulted in a significant decrease in stroke size and improvement in clinical outcome. Moreover, our analysis of human autoptic brain tissue found that the IL-17A signaling might also contribute to the pathophysiology in human stroke.
Inflammatory cascades in the course of the evolving ischemic brain damage are not restricted to resident immune cells of the brain but also involved recruited immune cells from the systemic immune compartment.3,16,29 Several mediators of these inflammatory cascades have previously been described. In stroke, overexpression of IFN-γ is detrimental,30 whereas neutralization of IFN-γ is rather protective.9 Furthermore, macrophage activation and TNF-α production are associated with cell death and induction of inflammation,31 but they have also been implicated in immune tolerance and repair, and in TNF-α–deficient animals cortical infarction and behavioral deficit are significantly exacerbated.32 Remarkably, we observe that microglia are not influenced by the presence of T cells or IFN-γ. This finding suggests that the microglial activation in the ischemic area relies primarily on damage-associated molecular patterns, which are released by dying cells.33 These damage-associated molecular patterns are recognized by pattern recognition and purinergic receptors, which are expressed on microglial cells, resulting in the rapid detection of the ischemic tissue damage.34 In contrast, the IFN-γ–dependent TNF-α production in macrophages suggests that the activation of macrophages in stroke exhibits similarities with a classic M1 activation, which requires a combination of TLR ligands and IFN-γ.35 The different mechanisms of activation could indicate a more protective function of microglia in stroke.32,36,37
As previously reported,7 we found that γδ T cells are a major source of IL-17A in postischemic tissue damage. γδ T cells can be activated merely by cytokines or danger signals, including IL-1β, IL-23, or TLR2 and dectin-1, respectively,15,38 and are, therefore, ideal candidates for a rapid activation in a setting of a sterile inflammation. This may explain why antigen-specific T-cell receptor activation is not necessary for early T cell–mediated damage in stroke.8 Neutrophils are a pathologic hallmark of early cerebral ischemia.2 IL-17A has been shown to attract neutrophils under ischemic conditions in kidney and lung,13,39 and our data clearly show that, in the absence of γδ T cells or IL-17A, the influx of neutrophils is markedly diminished in the ischemic brain. What triggers neutrophils to enter the ischemic brain tissue was unclear so far. This study shows that IL-17A secreted by γδ T cells in the presence of a local TNF-α rich milieu leads to a rapid up-regulation of CXCL-1, a neutrophil chemoattractant. The prominent role of CXCL-1 in neutrophil chemotaxis is underlined by reduced neutrophil infiltration after stroke in CXCR2-deficient mice. Even if the role of neutrophils in stroke is still controversial, the decrease in infarct size in MPO-deficient mice and in mice treated with neutralizing CXCR2 antiserum suggest a critical role for neutrophils. This observation is supported by findings in pharmacologic and transgenic mice models of neutrophil inhibition.40,41
In a therapeutic setting, neutralization of IL-17A even 3 hours after stroke resulted in diminished neutrophil infiltration and in a better neurologic outcome in mice. Thus, IL-17A provides an ideal target because its main appearance in stroke is short-lived and has, to our knowledge, only proinflammatory effects. Unlike IL-17A, neutralization of TNF-α or depletion of neutrophils will lead to multiple counterproductive or harmful effects. Remarkably, in addition to neutrophil infiltration, we observed early IL-17A production by T cells in human stroke tissue. Human clinical trials with humanized neutralizing IL-17A antibodies in patients with autoimmune disease have already provided favorable results.42,43
Unselective immunosuppression in stroke has harmful consequences. First, immunodepression after stroke leads to increased incidence of infections after stroke.29 Second, cells of the immune system, for example, microglia and regulatory T cells, are involved in neuroprotection37 or modulation of the immune response via secretion of IL-109,10 and TGF-β at later time points after stroke.12,39 Third, the immune system is also involved in tissue repair after stroke.3 Thus, neutralization of IL-17A might provide a novel selective therapeutic approach that targets proinflammatory cascades without affecting beneficial actions of immune cell subpopulations.
One specific possibility could be the combination of humanized neutralizing IL-17A antibodies with recombinant tissue plasminogen activator (rtPA), the only approved therapeutic option in patients who have had a stroke.44 Experimental data indicate that the activation of the immune system is a harmful side effect of rtPA.45,46 rtPA even induces MMP3 in endothelial cells, which we could show to be regulated by IL-17A in stroke.28 Combined application of rtPA together with a selective short lasting anti-inflammatory intervention such as neutralizing IL-17A antibody might not only reduce migration of neutrophils into the brain but also diminish additional proinflammatory effects of rtPA.
In conclusion, our study provides further insights into the inflammatory cytokine and immune cell networks after stroke and suggests that neutralizing IL-17A antibodies are a plausible therapeutic option in stroke.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Catherine Uyttenhove for providing the anti–IL-17A antibody, Stefanie Hünemörder for the preparation of the IL-17A antibody, and Theodore J. Standiford for providing the CXCR-2 goat/anti–mouse serum.
This work was supported by grants from the Landesexzellenzinitiative Hamburg (M.G., T.M., and C.G.) and ERANET/NANOSTROKE (T.M.).
Authorship
Contribution: M.G. conducted in vitro and in vivo experiments, analyzed data, and contributed to the design of the study and to the writing of the manuscript; A.W. conducted in vitro experiments and analyzed data; C.B., J.V., and M.G. conducted histologic immunochemical and immunofluorescence analysis and contributed to the writing of the manuscript; P.A. conducted in vivo experiments; K.S. and F.L. contributed to the writing of the manuscript and analysis of the FACS data; E.O., O.S., and V.T. conducted in vitro experiments; M.A.F., I.P., C.H., and C.G. contributed to the analysis of the data and the writing of the manuscript; T.V.A., T.K., and E.T. contributed to the analysis of the data, the design of the study and the writing of the manuscript; and T.M. contributed to the design of the study and the writing of the manuscript and supervised the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tim Magnus, Department of Neurology, University Medical Center Hamburg-Eppendorf, Martinistr 52, 20246 Hamburg, Germany; e-mail: t.magnus@uke.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal