Abstract
The pathophysiology of thrombotic thrombocytopenic purpura (TTP) can be explained by the absence of active ADAMTS13, leading to ultra-large von Willebrand factor (UL-VWF) multimers spontaneously interacting with platelets. Preventing the formation of UL-VWF–platelet aggregates therefore is an attractive new treatment strategy. Here, we demonstrate that simultaneous administration of the inhibitory anti-VWF monoclonal antibody GBR600 and the inhibitory anti-ADAMTS13 antibody 3H9 to baboons (prevention group) precluded TTP onset as severe thrombocytopenia and hemolytic anemia were absent in these animals. In addition, partial VWF inhibition was not enough to prevent thrombocytopenia, demonstrating the specificity of this therapeutic strategy. GBR600 treatment of baboons during acute TTP (treatment group) resulted in a rapid recovery of severe thrombocytopenia similar to the platelet count increases observed in TTP patients treated by plasma exchange. Baboons in the control group only injected with 3H9 developed early stages of TTP as previously described. Hence, inhibiting VWF-GPIb interactions is an effective way to prevent and treat the early symptoms of acquired TTP in baboons.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is largely characterized by a deficiency in the von Willebrand factor (VWF) cleaving protease ADAMTS13 leading to the presence of ultra-large (UL)–VWF multimers in patient plasma. These UL-VWF multimers cause spontaneous platelet aggregation through interaction with platelet glycoprotein (GP)Ib, thereby blocking capillaries and arterioles. Current standard treatment is replacement of the defective enzyme by plasma infusion or exchange.1 Because of the inherent risks and practical problems associated with plasma exchange, new treatment strategies for TTP are currently being explored.2 Because the presence of microthrombi cause thrombocytopenia, hemolytic anemia, and thrombosis, inhibition of microthrombus formation, using antibodies or DNA/RNA aptamers that block the GPIb-VWF interaction,3-6 might be a good alternative treatment approach. By administrating an inhibitory anti-ADAMTS13 antibody to baboons, we recently developed an animal model of acquired TTP7 that gives us the unique opportunity to perform a preclinical assessment of the usefulness of these new therapeutic strategies. In this study we investigated whether blocking VWF function using the inhibiting anti-VWF monoclonal antibody (mAb) GBR6006 resulted in the prevention or treatment of acquired TTP in baboons.
Methods
Cape baboons (Papio ursinus) were anesthetized by intramuscular delivery of 10 mg/kg ketamine hydrochloride. Injections and blood sampling were performed by venipuncture of the femoral vein while under anesthesia. Antibody 3H9 was injected at a dose of 600 μg/kg every 48 hours7 and GBR600 at a dose of 2 mg/kg every 24 hours (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). A full blood count and schistocytes were determined blindly.7 ADAMTS13 antigen and activity,7,8 VWF antigen,9 VWF:RCo activity,9 VWF multimer analysis,10 and concentration of mAbs 3H97 and GBR600 were determined as described. Data are represented as mean ± SEM. Statistics were performed using Student t test with Prism Version 5 software (GraphPad). Housing, treatment, phlebotomy, and care for the Cape baboons (P ursinus), as well as the final protocol were approved by the Interfaculty Animal Ethics Committee of the University of the Free State (Bloemfontein, South Africa) in accordance with the South African National Standard 10386: the use and care of animals for scientific purposes. For detailed materials and methods see supplemental Methods.
Results and discussion
Inhibition of VWF function prevents acquired TTP in baboons
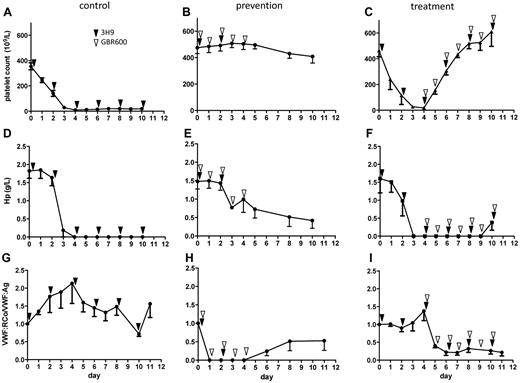
The inhibiting anti-VWF mAb GBR600 was injected together with the inhibiting anti-ADAMTS13 mAb 3H9 (supplemental Figure 1), to study whether blocking the VWF-GPIb interaction would prevent acquired TTP in baboons. TTP symptoms were absent in 3 of 4 animals treated with GBR600, whereas TTP symptoms clearly emerged in control animals not receiving any treatment (Figure 1). Indeed, inhibiting ADAMTS13 function by injecting 3H9 in control animals resulted in severe thrombocytopenia (Figure 1A), as previously described,7 whereas thrombocytopenia was prevented in 3 of 4 baboons when GBR600 was coadministered with 3H9 (Figure 1B). Also hemolytic anemia, measured by a decrease in hemoglobin (Hb; 14.0 ± 0.6 g/dL at day 0 and 11.7 ± 0.6 g/dL at day 10, P = .56, n = 3) and in haptoglobin (Hp) levels (Figure 1D) was observed in control baboons but not in the 3 baboons in the prevention group responding to treatment, as no changes were observed in Hb levels (13.6 ± 0.3 g/dL at day 0 and 12.9 ± 0.1 g/dL at day 10, P = NS, n = 3) and only minor changes in Hp levels (Figure 1E). The increase in number of schistocytes in the control group (from 0 at day 0 to 18.2 ± 5.1, at day 10, n = 3) was higher than in the 3 baboons in the prevention group (from 0 at day 0 to 6.9 ± 2.7 at day 10, n = 3). Control animals not injected with GBR600 had normal VWF activity levels throughout the study (Figure 1G), whereas injection of GBR600 during the first 5 days of the study resulted in maximal inhibition of ex vivo VWF binding to platelet GPIbα in 3 of 4 baboons in the prevention group (Figure 1H). UL-VWF multimers were present in plasmas from baboons in both the control and prevention group (supplemental Figure 2A-D) showing that binding of GBR600 to VWF in the prevention group neutralizes the harmful agglutinating effect of the UL-VWF multimers in these baboons.
Blocking the VWF-GPIb interaction prevents and treats acquired TTP in baboons. All baboons in the control (left panel), prevention (middle panel), and treatment (right panel) group received repeated injections of the inhibitory anti-ADAMTS13 mAb 3H9 (black arrow heads). Severe thrombocytopenia was observed in the control group (29 ± 5 × 109/L starting at day 3; A) together with hemolytic anemia evident from the severe decrease in Hp levels (1.8 ± 0.2 g/L at day 0 and 0 ± 0g/L at day 10, n = 3, P < .001; D). As baboons in the control group did not receive any GBR600, VWF activity (VWF:RCo/VWF:Ag) was not inhibited (G). Baboons in the prevention group were treated with GBR600 for 5 days (white arrow heads, middle panel). Platelet counts did not change (476 ± 39 × 109/L at day 0 and 408 ± 43 × 109/L at day 10, n = 3, B) and the decrease in Hp levels was moderate (1.5 ± 0.2 g/L at day 0 and 0.4 ± 0.2 g/L at day 10, P = .02, n = 3; E). Inhibition of VWF:RCo activity was observed when GBR600 was administered (H). Baboons in the treatment group (n = 3) received daily injections of GBR600 (right panel, white arrow heads) from day 4 onward. Severe thrombocytopenia was observed at day 3 and 4 but quickly normalized when GBR600 was injected (434 ± 25 × 109/L at day 7 versus 463 ± 48 × 109/L at day 0, P = NS, n = 3, C). Hemolytic anemia was evident from the severe decrease in Hp levels (1.6 ± 0.4 g/L at day 0 to 0 g/L at day 4, P = .01, n = 3; F). Injection of GBR600 from day 4 onward resulted in subsequent inhibition of VWF activity (I). Data are mean ± SEM, n = 3 in each group.
Blocking the VWF-GPIb interaction prevents and treats acquired TTP in baboons. All baboons in the control (left panel), prevention (middle panel), and treatment (right panel) group received repeated injections of the inhibitory anti-ADAMTS13 mAb 3H9 (black arrow heads). Severe thrombocytopenia was observed in the control group (29 ± 5 × 109/L starting at day 3; A) together with hemolytic anemia evident from the severe decrease in Hp levels (1.8 ± 0.2 g/L at day 0 and 0 ± 0g/L at day 10, n = 3, P < .001; D). As baboons in the control group did not receive any GBR600, VWF activity (VWF:RCo/VWF:Ag) was not inhibited (G). Baboons in the prevention group were treated with GBR600 for 5 days (white arrow heads, middle panel). Platelet counts did not change (476 ± 39 × 109/L at day 0 and 408 ± 43 × 109/L at day 10, n = 3, B) and the decrease in Hp levels was moderate (1.5 ± 0.2 g/L at day 0 and 0.4 ± 0.2 g/L at day 10, P = .02, n = 3; E). Inhibition of VWF:RCo activity was observed when GBR600 was administered (H). Baboons in the treatment group (n = 3) received daily injections of GBR600 (right panel, white arrow heads) from day 4 onward. Severe thrombocytopenia was observed at day 3 and 4 but quickly normalized when GBR600 was injected (434 ± 25 × 109/L at day 7 versus 463 ± 48 × 109/L at day 0, P = NS, n = 3, C). Hemolytic anemia was evident from the severe decrease in Hp levels (1.6 ± 0.4 g/L at day 0 to 0 g/L at day 4, P = .01, n = 3; F). Injection of GBR600 from day 4 onward resulted in subsequent inhibition of VWF activity (I). Data are mean ± SEM, n = 3 in each group.
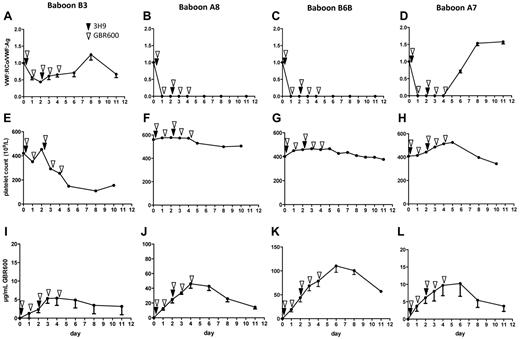
The observation that 1 of 4 baboons was not reacting to treatment seemed however logic as the VWF activity in this baboon was only moderately inhibited (baboon B3; Figure 2A), whereas the first injection of GBR600 in baboons A8, B6B, and A7 resulted in maximal ex vivo inhibition of VWF activity (Figure 2B-D). This low degree of VWF inhibition in baboon B3 correlated with the observed decrease in platelet counts (Figure 2E), which was not observed in baboons A8, B6B, and A7 (Figure 2F-H). Thrombocytopenia in baboon B3 was however less severe than in the control animals (Figure 2A vs Figure 1A).
The level of VWF inhibition correlates with platelet count. The first day after injection of GBR600 (open arrow heads), VWF activity was moderately inhibited in baboon B3 (A) and maximally inhibited in baboon A8 and B6B until day 11 (B-C) and in baboon A7 until day 4 (D). The moderate inhibition of VWF activity in baboon B3 was accompanied by a decrease in platelet counts (E), whereas platelet counts remained stable in baboons A8, BB, and A7 (F-H) when VWF activity was fully inhibited during the first 4 days of the experiment (B-D). GBR600 plasma levels were lower in baboon B3 (I) compared with baboons A8, B6B, and A7 (I-L). In every plasma sample, VWF:RCo/VWF:Ag levels (A-D), and GBR600 levels (I-L) were determined in triplicate and data are represented as mean ± SEM. Platelet counts were only measured once in each blood sample (E-H).
The level of VWF inhibition correlates with platelet count. The first day after injection of GBR600 (open arrow heads), VWF activity was moderately inhibited in baboon B3 (A) and maximally inhibited in baboon A8 and B6B until day 11 (B-C) and in baboon A7 until day 4 (D). The moderate inhibition of VWF activity in baboon B3 was accompanied by a decrease in platelet counts (E), whereas platelet counts remained stable in baboons A8, BB, and A7 (F-H) when VWF activity was fully inhibited during the first 4 days of the experiment (B-D). GBR600 plasma levels were lower in baboon B3 (I) compared with baboons A8, B6B, and A7 (I-L). In every plasma sample, VWF:RCo/VWF:Ag levels (A-D), and GBR600 levels (I-L) were determined in triplicate and data are represented as mean ± SEM. Platelet counts were only measured once in each blood sample (E-H).
Inhibition of VWF function reverses severe thrombocytopenia in acquired TTP in baboons
Injection of 3H9 in the treatment group (supplemental Figure 1 right panel) resulted in a fast and severe thrombocytopenia (Figure 1C, supplemental Figure 3A-C) as observed in the control group (Figure 1A). When daily injections of GBR600 were started in the treatment group at day 4, acquired TTP could be treated as a steady recovery of platelet counts was observed with a normalization already 3 days after GBR600 treatment initiation (Figure 1C, supplemental Figure 3A-C). In contrast, platelet counts in the control group not receiving GBR600 remained low until the end of the study (Figure 1A). Hemolytic anemia was also observed before GBR600 was administered in the treatment group as expected: Hp levels significantly dropped (Figure 1F) and Hb levels decreased from 12.2 ± 1.5 g/dL at day 0 to 9.8 ± 1.6 g/dL at day 4 (P = NS). Blocking VWF function by injecting GBR600 at day 4 resulted in a much slower recovery of hemolytic anemia (9.7 ± 1.4 g/dL Hb at day 10 and 0.4 ± 0.2 g/L Hp at day 10, P = .05, Figure 1F) compared with the recovery of the platelet count (Figure 1C), which is explained by the slower turnover of red blood cells. In line with this, schistocyte numbers remained high in the treatment group (from 0 at day 0 to 24 ± 9 at day 10, n = 3) as in the control group (from 0 at day 0 to 18.2 ± 5.1, at day 10, n = 3). A rapid inhibition of VWF function was detected as soon as GBR600 was administered at day 4 (Figure 1I, supplemental Figure 3D-F). Daily injections of GBR600 until the end of the study guaranteed continuous VWF inhibition (Figure 1I) because of accumulating GBR600 levels in baboon plasma (supplemental Figure 3G-I). UL-VWF multimers also persisted in the plasma of the baboons from the treatment group (supplemental Figure 2E-F).
The current treatment of choice for TTP is the replacement of the defective enzyme ADAMTS13 by plasma exchange or infusion or may be in the future by recombinant ADAMTS13.1,2 However, the outcome of the current study shows that inhibiting VWF-GPIb interaction both protects the baboons from acquired TTP (prevention group) as reflected by the absence of severe thrombocytopenia and treats the baboons during acquired TTP as evidenced by the rapid recovery of platelet counts (treated group; Figure 1). This treatment strategy was moreover specific as the degree of VWF inhibition adequately inversely correlates with the degree of thrombocytopenia (Figure 2). As the baboon model most probably represents the early stages of the disease, it remains to be determined whether this treatment strategy will also lead to clinical recovery when severe organ damage has occurred in the baboons, which corresponds to the clinical situation in TTP patients. However, data of clinical trials using inhibiting anti-VWF agents are encouraging as moderate increases in platelet counts were observed.11,12
In conclusion, the outcome of this study provides a further rationale to perform clinical trials using inhibitory VWF-GPIb compounds for the early treatment of TTP.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was funded by a Flemish FWO grant G.0584.11, a grant from the National Health Laboratory Service Research Trust, South Africa, and by Glenmark Pharmaceuticals S.A.
Authorship
Contribution: J.R. designed the study, provided the animals, performed experiments, analyzed data, and critically read the paper; H.B.F. provided essential reagents, designed the study, and critically read the paper; H.M. and S.H. provided essential reagents and critically read the paper; S.L., W.J.V.R., N.V., and I.P. performed experiments; H.D. critically read the paper; K.V. designed the study, provided essential reagents, performed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: H.M. and S.G. are employees of Glenmark Pharmaceuticals SA. The remaining authors declare no competing financial interests.
The current affiliation for J.R. is Transfusion Research Center, Belgian Red Cross, Flanders, Belgium.
Correspondence: Karen Vanhoorelbeke, Laboratory for Thrombosis Research, IRF Life Sciences, KU Leuven KULAK, E Sabbelaan 53, B-8500 Kortrijk, Belgium; e-mail: karen.vanhoorelbeke@kuleuven-kortrijk.be.
References
Author notes
H.B.F. and J.R. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal