Abstract
The interleukin-1 (IL-1) superfamily of cytokines comprises a set of pivotal mediators of inflammation. Among them, the action of IL-36 cytokines in immune responses has remained elusive. In a recent study, we demonstrated a direct effect of IL-36 on immune cells. Here we show that, among T cells, the IL-36 receptor is predominantly expressed on naive CD4+ T cells and that IL-36 cytokines act directly on naive T cells by enhancing both cell proliferation and IL-2 secretion. IL-36β acts in synergy with IL-12 to promote Th1 polarization and IL-36 signaling is also involved in mediating Th1 immune responses to Bacillus Calmette-Guerin infection in vivo. Our findings point toward a critical function of IL-36 in the priming of Th1 cell responses in vitro, and in adaptive immunity in a model of mycobacterial infection in vivo.
Introduction
The interleukin-1 (IL-1) family of cytokines comprises 11 members, including IL-1α, IL-1β, IL-18, IL-33, and the recently renamed IL-36α, β, γ (previously known as IL-1F6, IL-1F8, and IL-1F9).1 All these cytokines use heterodimeric receptors for signaling. IL-1, IL-33, and IL-36 bind to specific receptor α-chains, which are IL-1RI for IL-1α and IL-1β, T1/ST2 (also known as IL-33R) for IL-33, and IL-36R (previously termed IL-1Rrp2) for IL-36, and then recruit the same coreceptor IL-1R accessory protein (IL-1RAcP). IL-18 uses IL-18Rα and the coreceptor IL-18Rβ. On receptor binding, all IL-1 family cytokines activate similar intracellular signals, including NF-κB and mitogen-activated protein kinase (MAPK) pathways. IL-1 receptor antagonist (IL-1Ra) and IL-36Ra (previously termed IL-1F5), 2 additional members of the IL-1 family, act as natural inhibitors for the biologic activities of IL-1 and IL-36, respectively.2-4
IL-1α, IL-1β, IL-18, and IL-33 are produced by activated innate immune cells (neutrophils, monocytes, macrophages, and dendritic cells) and epithelial cells, and stimulate proinflammatory innate and adaptive immune responses. More specifically, these cytokines influence CD4+ T-cell responses and their polarization into the different T helper (Th) subsets Th1, Th2, and Th17. IL-1 promotes the proliferation and survival of naive CD4+ T cells and plays a critical role in Th17 differentiation.5-9 IL-18 and IL-33 stimulate the polarization of CD4+ T cells into Th1 and Th2, respectively,10 although the selectivity of these responses may be modulated by the cytokine environment. Consistently, Th1, Th2, and Th17 cells selectively express the IL-1 family cytokine receptors IL-18Rα, T1/ST2, and IL-1RI, respectively.10
IL-36 cytokines and IL-36R are abundantly expressed by keratinocytes and other epithelial cell types.4,11-13 IL-36 plays a major role in mouse experimental skin inflammation and in human psoriasis both in the initiation and regulation of inflammatory responses.14-19 Furthermore, the association of a form of generalized pustular psoriasis with genetic IL-36Ra deficiency in humans argues in favor of a significant role of IL-36 in inflammatory skin diseases.20,21 Recently, we have shown that dendritic cells (DCs) express IL-36R and that IL-36 stimulates the production of several cytokines and enhances the expression of costimulatory molecules in bone marrow–derived DCs (BMDCs). The stimulatory effects of IL-36 were more robust than those of the other members of the IL-1 family. In addition, IL-36 stimulated the production of interferon γ (IFN-γ), IL-4, and to a lesser extent IL-17 by cultured splenocytes and activated CD4+ T cells, and IL-36 was able to act as an adjuvant to stimulate Th1 responses in vivo.22
In the results described herein we show that, among CD4+ T-cell subsets, IL-36R is predominantly expressed by naive CD4+ T (referred to also as naive Th) cells and that IL-36 stimulates activated naive CD4+ T (referred to also as Th0) cell proliferation and IL-2 production, this effect being more potent than that of other IL-1 family cytokines. Furthermore, IL-36 acts in synergy with IL-12 to induce the polarization of Th0 cells into Th1 cells while endogenously produced IL-36β acts as an autocrine inducer of IFN-γ production in CD4+ T cells. Finally, IL-36 signaling was also critical for Th1-protective immune responses in an experimental model of Bacillus Calmette-Guerin (BCG) infection. These data support the hypothesis that IL-36 expressed both in epithelia and in immune cells may act as an early danger signal to activate cells of the innate and adaptive immunity such as DCs and naive CD4+ T cells to stimulate host responses against pathogens.
Methods
Reagents
Media used for mouse BMDCs and T-cell isolation and culture were obtained from Invitrogen Life Technologies. Recombinant mouse IL-33 was provided by Alexis Corporation, recombinant mouse IL-2 was purchased from PeproTech, recombinant human IL-1β, recombinant mouse IL-18 and recombinant mouse IL-12 were purchased from R&D Systems and purified LPS from Fluka (Escherichia coli 055:B5). Murine IL-36Ra, IL-36α, IL-36β, IL-36γ were produced at Amgen Inc as N-terminal truncation variants.23 The neutralizing anti–IL-2 mAb (IgG2a, clone S4B6) and anti-CD25 mAb (IgG1, clone PC61), as well as rat IgG2a isotype control Ab (clone R35-95) were purchased from BD Biosciences. Rat IgG1 isotype control Ab (clone M139) was produced at Amgen Inc. Concanavalin A (ConA) was purchased from Amersham Pharmacia Biotech (now part of GE Healthcare).
Mice
Wild-type (WT) C57BL/6J mice were obtained from Janvier. IL-36R–deficient mice (IL-136R−/−) were backcrossed 7 times into a pure C57BL/6J genetic background using a marker-assisted selection protocol (MASP) approach, also termed “speed congenics.”24 IL-12p35–deficient (IL-12p35−/−) and IL-2–deficient (IL-2−/−) mice in a pure C57BL/6J background were obtained from The Jackson Laboratory. All mice were maintained under conventional conditions in the animal facility of the Geneva University School of Medicine, and water and food were provided ad libitum. Animal studies were approved by the Animal Ethics Committee and the Geneva Veterinarian Office and were performed according to the appropriate codes of practice.
Purification and in vitro culture of naive T cells
Total CD4+ T cells were prepared from spleen by negative selection using immunomagnetic beads according to the manufacturer's protocol (CD4+ T Cell Isolation Kit II; Miltenyi Biotec) and passed through a magnetic cell sorting column (Miltenyi Biotec). CD62LhiCD44lo CD4+ T cells were purified from the CD4+ T cells by staining with PerCP-labeled rat anti-mouse CD4 (RM4-5), allophycocyanin-labeled rat anti–mouse CD62L (Ly-22, MEL-14), and PE-labeled rat anti–mouse CD44 (IM7; all from BD Biosciences) followed by sorting on a FacsVantage SE (BD Biosciences). The purity of naive CD4+CD62LhiCD44lo T-cell populations was usually > 95%. Th1, Th2, and Th17 were differentiated as described.22 CD4+CD62LhiCD44lo T cells (3 × 104 cells/well) were activated with plate-bound anti–mouse CD3/CD28 antibodies (0.5 μg/mL/0.5 μg/mL; BD Pharmingen) in flat-bottom 96-well plates in the presence of IL-36Ra, IL-36α, IL-36β, IL-36γ, IL-1β, IL-33, IL-18, IL-12, or LPS (100 ng/mL). In several experiments, activated naive T cells were cultured with IL-36β (100 ng/mL), IL-12 (10 ng/mL), and IL-18 (100 ng/mL), alone or in different combinations. Where specified, IL-2 (10 ng/mL), neutralizing anti–IL2, and anti-CD25 mAbs (10 μg/mL) or isotype control mAb (10 μg/mL) were used. Cytokines were incubated 1 hour in the presence of anti–IL-2 mAb or its isotype control mAb prior the addition to cell cultures. In the meantime, the cells were incubated with anti-CD25 mAb or its isotype control mAb for 1 hour before the addition of the stimulants. Anti–IL2 antibody (10 μg/mL) or its isotype control were added again after 15 hours. After 2 days of stimulation, cell supernatants were collected and cytokine (IL-2, IFN-γ) levels were measured by ELISA (R&D Systems and eBioscience).
Generation of BMDCs and coculture with naive T cells
Details of these procedures are available in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Proliferation assay
Naive T cells were cultured (4 × 104 cells/ well) in a 96-well plate precoated with anti-CD3(0.5 μg/mL)/anti-CD28 mAb (0.5 μg/mL) and incubated at 37°C with 5% CO2 for 72 hours with or without 100 ng/mL recombinant IL-36R ligands, IL-1β, IL-33, IL-18, IL-12, or LPS. Cell proliferation was assessed by measuring the uptake of [3H]-thymidine (1 μCi/well) during the last 18 hours of culture. Cells were harvested onto glass-fiber filters (PerkinElmer), and retained radioactivity was measured in a liquid scintillation counter. Mean counts per minute (cpm) ± SD were calculated from triplicate wells. In parallel, in another plate, cell-culture supernatants were harvested for cytokine assays.
Flow cytometry and determination of surface and intracellular cytokine staining
Fluorochrome-labeled mAbs specific for CD11c (HL3), CD11b (M1/70), CD4 (RM4-5), CD62L (MEL-14), and CD44 (IM7) were obtained from Biosciences. For cell-surface staining, cells were preincubated with mAb 2.4G2 (anti-CD16/32) to block Fc receptors, and labeled with mAbs in PBS, 0.2% BSA, 10mM EDTA. Labeled cells were run on a FACSCalibur, and data were analyzed using the CellQuest software. Detection of IFN-γ–producing cells was performed by intracellular cytokine staining with anti–IFN-γ–FITC (BD Biosciences). In brief, cells (1.5 × 105 cells/well) were stimulated with 10 ng/mL phorbol myristate acetate (PMA) and 500 ng/mL ionomycin in the presence of Golgistop (BD Biosciences) for 4 hours at 37°C. Cells were then fixed and permeabilized with Cytofix/Cytoperm Plus (BD Biosciences) according to the manufacturer's instructions. Cells were incubated with FITC-labeled rat anti–IFN-γ mAb in 1× PBS 0.2% BSA, washed, and data were acquired on a FACSCalibur (BD Biosciences). Cell-culture supernatants were collected for cytokine assays.
CFSE labeling
For CFSE labeling, sorted naive CD4+CD44lowCD62Lhigh T cells were washed twice in staining buffer (PBS0.1% BSA), resuspended in staining buffer containing 5μM CFSE (Molecular Probes), and incubated at 37°C for 10 minutes. Five volumes of ice-cold culture medium were added to stop labeling, and cells were washed 3 times with culture medium. Cells were then activated with plate-bound anti–mouse CD3 and CD28 antibodies and stimulated with or without IL-36β (100 ng/mL), IL-12 (10 ng/mL), IL-18 (100 ng/mL), and IL-2 (10 ng/mL), alone or in different combinations. Where specified, neutralizing anti–IL-2 and anti-CD25 mAbs (10 μg/mL) or isotype control mAbs (10 μg/mL) were used as detailed in the previous paragraph. After 3 days of incubation, cell division was determined by measuring CFSE fluorescence of 7-amino actinomycin D (7-AAD; BD Pharmingen) negative–gated cells on a FACSCalibur (BD Biosciences). Data were analyzed using the CellQuest software. Cell-culture supernatants were collected for cytokine assays.
Real-time quantitative RT-PCR
Total RNA was extracted using the TRIzol reagent (Invitrogen Life Technologies) according to the manufacturer's protocol. Total RNA (1 μg) was reverse transcribed using SuperScript II Reverse transcriptase (Invitrogen Life Technologies). The mRNA levels for genes of interest were examined by quantitative RT-PCR using SYBR Green PCR Master Mix (Applied Biosystems). All primers sequences are described in supplemental Methods.
Values obtained with the SDS 2.2 (Applied Biosystems) were imported into Microsoft Excel for analyses and gene expression was calculated using the comparative method (2−ΔCt) for relative quantification by normalization to GAPDH gene expression.
Mycobacterium bovis BCG infection, lung bacterial burden, and ex vivo restimulation
Eight-week-old WT and IL-36R−/− mice were infected intravenously (IV) with 5 × 105 living M bovis BCG Connaught as previously described.25 Mice were killed 4 weeks after infection. Lung bacterial load was evaluated by plating lung homogenates on 7H11 agar plates and counting the number of colonies 3-4 weeks after. Cells of individual mice were isolated from spleen, plated (5 × 105 cells/well) and stimulated ex vivo with either medium alone, living M bovis BCG (103 CFU/well), or with antigens derived from M bovis BCG (1.7 μg/mL or 17 μg/mL).26 After 72 hours, the culture supernatants were harvested and tested by ELISA or by the multiplex bead-based immunoassay kit (Merck Millipore) according to the manufacturer's protocol. Accumulation of nitrite in the medium was measured as previously described.27
Histologic analyses
Histologic analyses of lung lesions were performed 4 weeks after infection. Lungs were fixed in 4% buffered formaldehyde and embedded in paraffin for subsequent hematoxylin/eosin (H&E) staining. Evaluation of the lesions was performed on at least 3 lobe sections per animal. Lung sections were captured on a Zeiss Mirax Scan microscope system. Virtual sections were subdivided and images (40-200 depending on the surface) processed for quantification of free and occupied space (lung tissue), as well as of the cellular content (nuclei) using specific programs in the Metamorph software.
Statistical analysis
One-way analysis of variance (ANOVA) and the 2-tailed t test were used for statistical analysis. A comparison between 2 groups using the 2-tailed t test was made only when the 1-way ANOVA yielded statistically significant results. P values of .05 or less were considered significant.
Results
IL-36R is highly expressed in naive Th cells
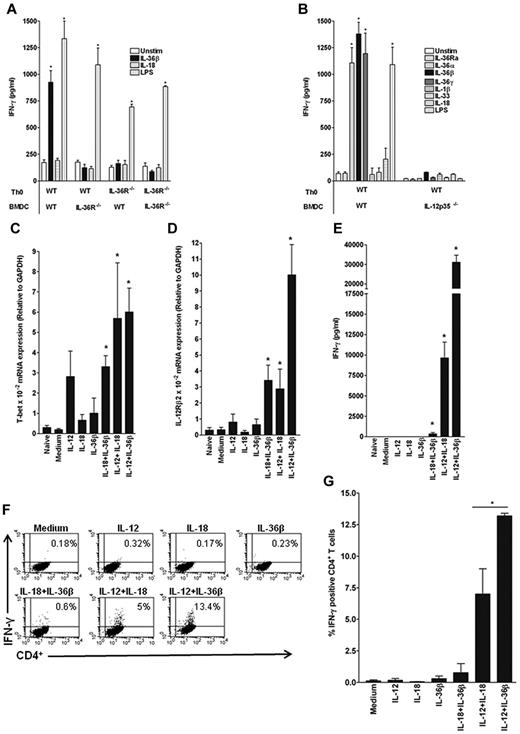
We previously described high expression of IL-36R mRNA in BMDCs and splenic CD4+ T cells.22 To extend our analyses, we examined the expression profile of IL-36R mRNA, as well as other IL-1 family receptors, in different CD4+ T-cell subsets. We observed that IL-36R was highly expressed in naive Th cells compared with Th1, Th2, or Th17 cells (Figure 1 A). Consistent with previous findings, IL-18Rα, T1/ST2, and IL-1R1 were virtually absent in Th0 cells and selectively up-regulated in Th1, Th2, and Th17 CD4+ T cells, respectively (Figure 1B-D). To investigate whether IL-36R was functional, naive Th cells were cultured in the presence of anti-CD3 and anti-CD28 antibodies in the absence or presence of IL-36Ra, IL-36α, IL-36β, or IL-36γ and of IL-1β, IL-33, IL-18, IL-12, or LPS as controls. Consistent with the pattern of receptor expression, IL-36R agonists, but not other IL-1 family cytokines or LPS, significantly stimulated IL-2 secretion (Figure 1E, P < .05) and cell proliferation (Figure 1F, P < .05) in WT, but not in activated IL-36R−/− Th0 cells. Thus, IL-36R is selectively expressed on naive Th cells and when triggered by its specific ligands, potently induces IL-2 production and cell division.
IL-36R is highly expressed and functional in naive CD4+ T cells. CD44loCD62Lhi naive CD4+ T cells (Th0 cells) were FACS sorted and polarized or not under Th1, Th2, or Th17 cell conditions for 5 days. Total mRNA was isolated from naive Th cells directly after the sort and from Th1, Th2, and Th17 differentiated cells for analyses by quantitative RT-PCR. Results represent (A) IL-36R, (B) IL-1R1, (C) IL-18Rα, and (D) ST2 mRNA expression levels relative to GAPDH. Error bars represent the SD of the mean of 3 independent experiments. (E-F) Naive T cells from WT and IL-36R−/− mice (4 × 104 cells per well) were sorted and cultured in 96-well plates precoated with anti-CD3 and anti-CD28 mAb and incubated for 72 hours in the absence (Medium) or presence of IL-36R ligands, IL-1β, IL-33, IL-18, IL-12, or LPS (100 ng/mL). (E) IL-2 secretion in culture supernatants was determined by ELISA. (F) Proliferative responses were assessed by [3H]-thymidine incorporation. Data are shown from one of 3 independent experiments with similar results. Error bars represent the SD of triplicates in the same experiment. *P < .05 (Student t test); IL-36R agonist stimulation significantly differs from unstimulated cells.
IL-36R is highly expressed and functional in naive CD4+ T cells. CD44loCD62Lhi naive CD4+ T cells (Th0 cells) were FACS sorted and polarized or not under Th1, Th2, or Th17 cell conditions for 5 days. Total mRNA was isolated from naive Th cells directly after the sort and from Th1, Th2, and Th17 differentiated cells for analyses by quantitative RT-PCR. Results represent (A) IL-36R, (B) IL-1R1, (C) IL-18Rα, and (D) ST2 mRNA expression levels relative to GAPDH. Error bars represent the SD of the mean of 3 independent experiments. (E-F) Naive T cells from WT and IL-36R−/− mice (4 × 104 cells per well) were sorted and cultured in 96-well plates precoated with anti-CD3 and anti-CD28 mAb and incubated for 72 hours in the absence (Medium) or presence of IL-36R ligands, IL-1β, IL-33, IL-18, IL-12, or LPS (100 ng/mL). (E) IL-2 secretion in culture supernatants was determined by ELISA. (F) Proliferative responses were assessed by [3H]-thymidine incorporation. Data are shown from one of 3 independent experiments with similar results. Error bars represent the SD of triplicates in the same experiment. *P < .05 (Student t test); IL-36R agonist stimulation significantly differs from unstimulated cells.
IL-36β synergizes with IL-12 to promote Th1 differentiation
We previously observed that IL-36 induces the production of various cytokines by BMDCs in vitro and stimulates Th1 responses in vivo.22 We thus examined a potential role of IL-36 cytokines in the polarization of naive T cells using a coculture system whereby naive Th cells were activated by anti-CD3 and anti-CD28 antibodies in the presence of BMDCs with or without IL-36R ligands, IL-1β, IL-33, IL-18, or LPS (Figure 2B). IL-36β strongly enhanced IFN-γ production by WT Th0 cells cultured in the presence of BMDCs to an extent comparable with that induced by LPS. Interestingly, IL-36R deficiency on either Th0 cells and/or BMDCs resulted in a complete loss of IFN-γ production in response to IL-36β (Figure 2A). In these cultures, IL-18 had no significant effect on IFN-γ production (Figure 2A) while IL-17 and IL-4 were never detected (data not shown).
Importance of IL-12 for the effect of IL-36 on Th1 differentiation. FACS-sorted naive CD4+T cells from WT (A-B) or IL-36R−/− (A) mice were activated with anti-CD3/anti-CD28 mAbs and cocultured with BMDCs from either WT (A-B), IL-36R−/− (A), or IL-12p35−/− (B) mice in the absence or presence of IL-36R ligands, IL-1β, IL-33, IL-18, and LPS (100 ng/mL). Three days later, IFN-γ was measured in the supernatant by ELISA (A-B). Data are shown from one of 3 independent experiments with similar results. Error bars represent the SD of triplicates in the same experiment. *P < .05 (Student t test), IL-36, IL-1β, IL-33, IL-18, or LPS stimulation significantly differs from unstimulated cells. (C-D) FACS-sorted naive CD4+ T cells were activated (Medium) or not (Naive) with plate-bound anti-CD3/anti-CD28 mAbs and stimulated for 72 hours with IL-12 (10 ng/mL), IL-18 (100 ng/mL), IL-36β (100 ng/mL) either alone or in various combinations as indicated. Total mRNA was isolated for analyses by quantitative RT-PCR. Results represent T-bet (C) and IL-12Rβ2 (D) mRNA expression levels relative to GAPDH. Error bars represent the SD of the mean of 3 independent experiments. *P < .05 (Student t test), IL-36, IL-12, and IL-18 stimulation alone or in combination significantly differs from unstimulated cells. (E) IFN-γ secretion was measured in culture supernatants by ELISA. Data are shown from one of 3 independent experiments with similar results. Error bars represent SD of triplicates in the same experiment. *P < .05 (Student t test), IL-36, IL-12, and IL-18 stimulation alone or in combination significantly differs from unstimulated cells. (F) Flow cytometric analysis of intracellular staining of IFN-γ in CD4+ T cells obtained from WT mice and cultured for 3 days with priming (anti-CD3/anti-CD28 mAbs) and different stimulations as indicated. FACS profiles shown in panel F represent one of 3 independent experiments with similar results for which quantitative data are shown in panel G. (G) Results represent the percentage of IFN-γ–positive CD4+ T cells for each condition. Error bars represent the SD of the mean of 3 independent experiments. *P < .05 (Student t test), percentage of IFN-γ–positive CD4+ T cells after IL-12/IL-18 stimulation significantly differs from IL-12/IL-36β stimulation.
Importance of IL-12 for the effect of IL-36 on Th1 differentiation. FACS-sorted naive CD4+T cells from WT (A-B) or IL-36R−/− (A) mice were activated with anti-CD3/anti-CD28 mAbs and cocultured with BMDCs from either WT (A-B), IL-36R−/− (A), or IL-12p35−/− (B) mice in the absence or presence of IL-36R ligands, IL-1β, IL-33, IL-18, and LPS (100 ng/mL). Three days later, IFN-γ was measured in the supernatant by ELISA (A-B). Data are shown from one of 3 independent experiments with similar results. Error bars represent the SD of triplicates in the same experiment. *P < .05 (Student t test), IL-36, IL-1β, IL-33, IL-18, or LPS stimulation significantly differs from unstimulated cells. (C-D) FACS-sorted naive CD4+ T cells were activated (Medium) or not (Naive) with plate-bound anti-CD3/anti-CD28 mAbs and stimulated for 72 hours with IL-12 (10 ng/mL), IL-18 (100 ng/mL), IL-36β (100 ng/mL) either alone or in various combinations as indicated. Total mRNA was isolated for analyses by quantitative RT-PCR. Results represent T-bet (C) and IL-12Rβ2 (D) mRNA expression levels relative to GAPDH. Error bars represent the SD of the mean of 3 independent experiments. *P < .05 (Student t test), IL-36, IL-12, and IL-18 stimulation alone or in combination significantly differs from unstimulated cells. (E) IFN-γ secretion was measured in culture supernatants by ELISA. Data are shown from one of 3 independent experiments with similar results. Error bars represent SD of triplicates in the same experiment. *P < .05 (Student t test), IL-36, IL-12, and IL-18 stimulation alone or in combination significantly differs from unstimulated cells. (F) Flow cytometric analysis of intracellular staining of IFN-γ in CD4+ T cells obtained from WT mice and cultured for 3 days with priming (anti-CD3/anti-CD28 mAbs) and different stimulations as indicated. FACS profiles shown in panel F represent one of 3 independent experiments with similar results for which quantitative data are shown in panel G. (G) Results represent the percentage of IFN-γ–positive CD4+ T cells for each condition. Error bars represent the SD of the mean of 3 independent experiments. *P < .05 (Student t test), percentage of IFN-γ–positive CD4+ T cells after IL-12/IL-18 stimulation significantly differs from IL-12/IL-36β stimulation.
Because we previously reported that IL-12 production was strongly stimulated in BMDCs by IL-36R agonists and to establish the role of IL-12 in the induction of IFN-γ in our coculture system, we cocultured WT Th0 cells with either WT or IL-12p35−/− BMDCs. Production of IFN-γ in response to IL-36 was completely abrogated when using IL-12p35−/− BMDCs (Figure 2B), indicating that IL-12 production by BMDCs is necessary for IL-36 to exert its stimulatory effect on IFN-γ in this coculture system. Consistent with the critical role of IL-12 in Th1 polarization, LPS also failed to induce IFN-γ production in the absence of IL-12p35 (Figure 2B). Stimulation of WT or IL-12p35−/− BMDCs with IL-36 resulted in similar IL-6 production (data not shown), thus ruling out a possible impaired IL-36 responsiveness in IL-12p35−/− BMDCs.
To determine whether IL-12 production by BMDCs was sufficient to allow Th1 polarization in response to IL-36 or whether additional soluble or cell-bound signals were required, we used an Ag-presenting cell–free system and stimulated Th0 cells with anti-CD3/anti-CD28 antibodies in the presence of recombinant cytokines. The combinations of IL-12 + IL-18, which is known to drive differentiation into Th1 cells, as well as of IL-12 + IL-36β both induced T-bet and IL-12Rβ2 mRNA expression (Figure 2C-D). Interestingly, however, IL-12Rβ2 mRNA levels were much higher when Th0 cells were primed in the presence of IL-12 + IL-36β rather than IL-12 + IL-18, pointing toward a potentially more efficient cooperation between IL-36 and IL-12 than between IL-18 and IL-12 signaling in T cells. Indeed, the combination of IL-12 and IL-36β strongly stimulated production of IFN-γ by Th0 cells, and was more efficient in doing so than the combination of IL-12 and IL-18 (Figure 2E). The increased IFN-γ secretion induced by IL-12 + IL-36 was because of an increase in the percentage of IFN-γ–producing cells (Figure 2F-G), although it may also in part result from an increase in the total cell number because of the IL-36 effect on T-cell proliferation (Figure 1F). None of the cytokines tested, when used alone, was sufficient to induce differentiation of Th0 cells into IFN-γ–producing Th1 cells (Figure 2E-G). Moreover, the combination of IL-18 + IL-36 only very moderately induced IFN-γ production. Taken together, these data clearly show that the presence of IL-12 is sufficient to allow a Th1 polarizing effect of IL-36 and that like IL-18, IL-36β acts as a copolarization factor with IL-12 to up-regulate expression of Th1-associated genes in activated Th0 cells. Furthermore, our data suggest that the synergistic effect of IL-12 + IL-36 stimulation on IL-12Rβ2 contributes to the amplification of the IL-12 signal.
IL-2 is necessary for the IL-36–induced Th1 polarization but only partially required for IL-36–induced cell proliferation and survival
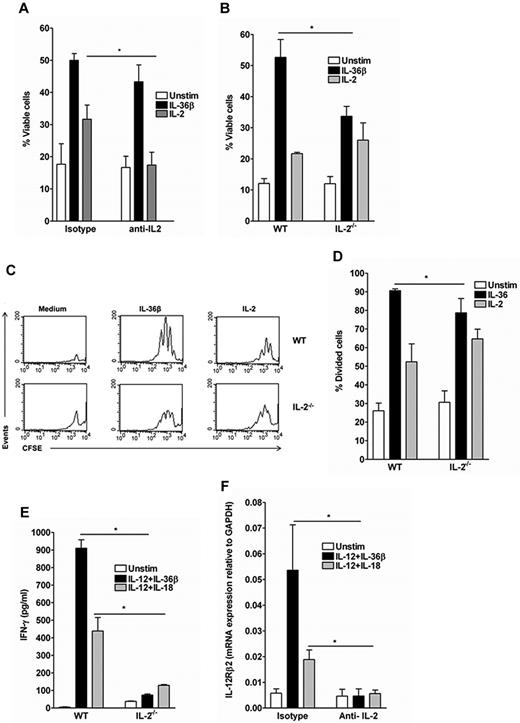
IL-2 is a potent survival and T-cell growth factor.28-31 Because IL-36 was a potent inducer of IL-2 production by Th0 cells (Figure 1), we investigated whether the stimulatory effect of IL-36β on both cell proliferation and Th1 differentiation was dependent on IL-2. WT and IL-2−/− naive T cells were labeled with CFSE, activated with anti-CD3 plus anti-CD28 and stimulated or not for 72 hours with IL-36β or IL-2 (Figure 3B-E). In WT naive T cells, IL-2 neutralization conditions were investigated using blocking anti-CD25 and anti-IL-2 mAbs (Figure 3A,F). The percentage of viable 7AAD− CD4+ T cells was markedly increased in the presence of IL-36β or IL-2, representing 50% ± 3.6% and 30% ± 7.6% of total cells, respectively, compared with 18% ± 8.5% in unstimulated cell cultures (Figure 3A). The viability of T cells in IL-36β–stimulated cultures was not altered in IL-2–neutralizing conditions (45% ± 9% vs 50% ± 3.6%), whereas the percentage of viable cells in IL-2–stimulated cultures was comparable with that of unstimulated cells (18% ± 7% vs 17% ± 6%). There were also no significant differences in the viability of cells stimulated with IL-12 + IL-36β or IL-12 + IL-18 with or without IL-2 neutralization (data not shown). We then used IL-2−/− naive T cells to further examine the role of IL-2 on IL-36 stimulatory activity. IL-36β significantly increased the percentage of cell viability (33.6% ± 5.5% compared with 12% ± 4%) in IL-2−/− naive T cells, but to a significantly lower extent than in WT Th0 cells (52.7% ± 9%; Figure 3B). We next investigated the effects of IL-36β on T-cell proliferation and found that IL-36β–stimulated T cells underwent the same number of cell divisions, as assessed by CFSE dilution, and accumulated to the same extent in IL-2−/− and WT Th0 cells, although the proliferation was significantly lower in IL-2−/− than in WT Th0 cells (Figure 3C-D).
Different contributions of IL-2 signaling to the effect of IL-36β on Th0 cell survival, proliferation, and Th1 polarization. FACS-sorted naive WT and IL-2−/− CD4+T cells were labeled with CFSE, activated with plate-bound anti-CD3/anti-CD28 mAb, and stimulated or not for 72 hours with IL-36β, IL-12 + IL-36β, IL-12 + IL-18 or IL-2. When specified, WT CD4+T cells where cultured in presence of blocking anti-CD25 and anti-IL-2 mAbs or isotype-matched control mAbs. (A-B) FACS analyses of viable 7AAD− CD4+ T cells. Error bars represent the SD of the mean of 3 independent experiments. *P < .05 (Student t test), cytokine stimulation in IL-2−/− or in presence of neutralizing treatment significantly differs from WT or isotype control treatment, respectively. (C-D) Analysis of cell divisions in WT and IL-2−/− Th0 cells. (C) Histogram profiles of CFSE-labeled cells. Data shown are representative of one of 3 independent experiments with similar results. (D) Percentage of divided cells as assessed by CSFE labeling obtained in panel C. Error bars represent the SD of the mean of 3 independent experiments.*P < .05 (Student t test), cytokine stimulation in WT Th0 cells significantly differs from the same stimulation in IL2−/− Th0 cells. (E) IFN-γ secretion in WT and IL-2−/− naive CD4+ T-cell supernatants after 72 hours of stimulation with IL-12 + IL-18 and IL-12 + IL-36β. Data are shown from one of 3 independent experiments with similar results. Error bars represent SD of triplicates in the same experiment. *P < .05 (Student t test), cytokine stimulation in WT naive CD4+ T cells significantly differs from the same stimulation in IL-2−/− naive CD4+ T cells. (F) Quantitative RT-PCR analysis of IL-12Rβ2 mRNA expression in WT naive CD4+ T cells stimulated or not for 72 hours with IL-12 + IL-18 and IL-12 + IL-36β in presence of blocking anti-CD25 and anti-IL-2 mAbs or isotype-matched control mAbs. Error bars represent the SD of the mean of 3 independent experiments. *P < .05 (Student t test), cytokine stimulation in presence of neutralizing treatment significantly differs from isotype control treatment
Different contributions of IL-2 signaling to the effect of IL-36β on Th0 cell survival, proliferation, and Th1 polarization. FACS-sorted naive WT and IL-2−/− CD4+T cells were labeled with CFSE, activated with plate-bound anti-CD3/anti-CD28 mAb, and stimulated or not for 72 hours with IL-36β, IL-12 + IL-36β, IL-12 + IL-18 or IL-2. When specified, WT CD4+T cells where cultured in presence of blocking anti-CD25 and anti-IL-2 mAbs or isotype-matched control mAbs. (A-B) FACS analyses of viable 7AAD− CD4+ T cells. Error bars represent the SD of the mean of 3 independent experiments. *P < .05 (Student t test), cytokine stimulation in IL-2−/− or in presence of neutralizing treatment significantly differs from WT or isotype control treatment, respectively. (C-D) Analysis of cell divisions in WT and IL-2−/− Th0 cells. (C) Histogram profiles of CFSE-labeled cells. Data shown are representative of one of 3 independent experiments with similar results. (D) Percentage of divided cells as assessed by CSFE labeling obtained in panel C. Error bars represent the SD of the mean of 3 independent experiments.*P < .05 (Student t test), cytokine stimulation in WT Th0 cells significantly differs from the same stimulation in IL2−/− Th0 cells. (E) IFN-γ secretion in WT and IL-2−/− naive CD4+ T-cell supernatants after 72 hours of stimulation with IL-12 + IL-18 and IL-12 + IL-36β. Data are shown from one of 3 independent experiments with similar results. Error bars represent SD of triplicates in the same experiment. *P < .05 (Student t test), cytokine stimulation in WT naive CD4+ T cells significantly differs from the same stimulation in IL-2−/− naive CD4+ T cells. (F) Quantitative RT-PCR analysis of IL-12Rβ2 mRNA expression in WT naive CD4+ T cells stimulated or not for 72 hours with IL-12 + IL-18 and IL-12 + IL-36β in presence of blocking anti-CD25 and anti-IL-2 mAbs or isotype-matched control mAbs. Error bars represent the SD of the mean of 3 independent experiments. *P < .05 (Student t test), cytokine stimulation in presence of neutralizing treatment significantly differs from isotype control treatment
IL-2 is also known to directly up-regulate IFN-γ mRNA expression in T cells and to synergize with IL-12 to promote Th1 differentiation.28,29,32 Thus, we investigated the effect of IL-12 + IL-36β and IL-12 + IL-18 on IFN-γ production in IL-2−/− T cells and in IL-2–neutralizing conditions. IL-12 + IL-36β– and IL-12 + IL-18–induced IFN-γ production was significantly reduced in IL-2−/− compared with WT Th0 cells (Figure 3E). Similarly, IFN-γ production (data not shown) and IL-12Rβ2 mRNA expression were markedly inhibited in IL-2–neutralizing conditions (Figure 3F), indicating that the effect of IL-36β on Th1 polarization was entirely dependent of IL-2. All together, these data demonstrate that IL-2 is necessary to mediate the effects of IL-36 on Th1 polarization, whereas only partially required in the IL-36–induced enhancement of T-cell proliferation and survival.
Endogenous IL-36 is required for efficient Th1 polarization in vitro and in vivo
In our previous study, we found that in BMDCs, IL-36γ mRNA was constitutively expressed, while IL-36α mRNA could be induced by innate signals.22 IL-36Ra or IL-36β mRNAs were never detected, at least in the stimulatory conditions tested. The same analysis in naive T cells revealed that IL-36β (Figure 4A) was constitutively expressed, while IL-36α, IL-36γ, or IL-36Ra were not expressed nor could they be induced by activation (data not shown), indicating a cell-specific distribution of IL-36 cytokine expression in immune cells.
Importance of endogenous IL-36 in the Th1 response. (A) FACS-sorted naive CD4+ T cells were activated (Medium) or not (Naive) with plate-bound anti-CD3 and anti-CD28 mAb and stimulated with IL-12 (10 ng/mL), IL-18 (100 ng/mL), IL-36β (100 ng/mL) either alone or in various combinations as indicated for 72 hours. Total mRNA was isolated from naive T cells for analyses by quantitative RT-PCR. Results represent IL-36β mRNA expression levels relative to GAPDH. Error bars represent the SD of the mean of 3 independent experiments. (B-C) IL-2 (B) and IFN-γ (C) production profiles in WT and IL-36R−/− naive CD4+T cells after different stimulations as indicated. Data are shown from one of 3 independent experiments with similar results. Error bars represent SD of triplicates in the same experiment. *P < .05 (Student t test), IL-12 + IL-18 stimulation in WT Th0 cells significantly differs from the same stimulation in IL-36R−/− Th0 cells. (D-E) Activated WT and IL-36R−/− Th0 cells were stimulated or not with IL-12/IL-18 and IL-12/IL-36β combinations and then treated with PMA plus ionomycin for 5 hours. IFN-γ–expressing CD4+ T cells were detected by intracellular staining and quantified by flow cytometry. FACS profiles shown in panel D represent one of 3 independent experiments with similar results for which quantitative data are shown in panel E. (E) Results represent the percentage of IFN-γ+CD4+ T cells for each condition. Error bars represent the SD of the mean of 3 independent experiments. *P < .05 (Student t test), IL-12 + IL-18 stimulation in WT cells significantly differs from the same stimulation in IL-36R−/− cells.
Importance of endogenous IL-36 in the Th1 response. (A) FACS-sorted naive CD4+ T cells were activated (Medium) or not (Naive) with plate-bound anti-CD3 and anti-CD28 mAb and stimulated with IL-12 (10 ng/mL), IL-18 (100 ng/mL), IL-36β (100 ng/mL) either alone or in various combinations as indicated for 72 hours. Total mRNA was isolated from naive T cells for analyses by quantitative RT-PCR. Results represent IL-36β mRNA expression levels relative to GAPDH. Error bars represent the SD of the mean of 3 independent experiments. (B-C) IL-2 (B) and IFN-γ (C) production profiles in WT and IL-36R−/− naive CD4+T cells after different stimulations as indicated. Data are shown from one of 3 independent experiments with similar results. Error bars represent SD of triplicates in the same experiment. *P < .05 (Student t test), IL-12 + IL-18 stimulation in WT Th0 cells significantly differs from the same stimulation in IL-36R−/− Th0 cells. (D-E) Activated WT and IL-36R−/− Th0 cells were stimulated or not with IL-12/IL-18 and IL-12/IL-36β combinations and then treated with PMA plus ionomycin for 5 hours. IFN-γ–expressing CD4+ T cells were detected by intracellular staining and quantified by flow cytometry. FACS profiles shown in panel D represent one of 3 independent experiments with similar results for which quantitative data are shown in panel E. (E) Results represent the percentage of IFN-γ+CD4+ T cells for each condition. Error bars represent the SD of the mean of 3 independent experiments. *P < .05 (Student t test), IL-12 + IL-18 stimulation in WT cells significantly differs from the same stimulation in IL-36R−/− cells.
The constitutive endogenous expression of IL-36β in naive T cells prompted us to investigate the role of IL-36 in Th1 polarization induced by IL-12 and IL-18. Strikingly, we found that IL-36R−/− Th0 cells stimulated with IL-12 and IL-18 were severely impaired in their ability to secrete IL-2 and IFN-γ compared with IL-36R+/+ Th0 cells (Figure 4B-C). Similarly, the increased percentage of IFN-γ–producing cells induced by IL-12 + IL-18 was strictly dependent on IL-36R expression in Th0 cells (Figure 4D-E). These results suggest the existence of a positive autocrine IL-36/IL-36R amplification loop, enhancing cell proliferation and Th1 polarization.
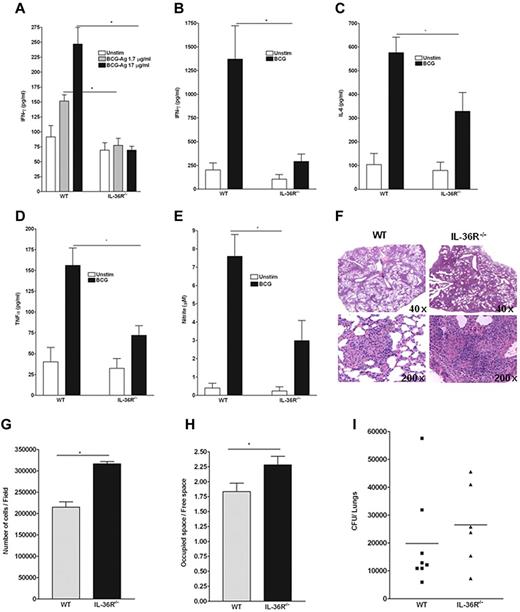
To address the contribution of endogenous IL-36 signaling to immune responses in vivo, WT and IL-36R−/− mice were infected with M bovis BCG. After 4 weeks splenocytes were isolated and restimulated ex vivo with antigens derived from M bovis BCG (Figure 5A) or with viable M bovis BCG (Figure 5B-E) and the levels of proinflammatory cytokines were determined in culture supernatants (Figure 5A-E). The production of IFN-γ after antigen-specific or living M bovis stimulation of splenocytes was significantly impaired in IL-36R−/− compared with WT mice (Figure 5A-B). These results suggest a deficiency in antigen-specific T-cell activation in IL-36R−/− compared with WT mice. In addition, the production of IL-6, TNF-α, and nitrite was also decreased in IL-36R−/− compared with WT splenocytes (Figure 5C-E). In contrast, IL-10 production, which was similarly induced by BCG stimulation, was not different in IL-36R−/− and WT splenocytes (data not shown), while the levels of other cytokines, including IL-12p70, IL-4, IL-13, and IL-17 were below the detection limit of the assay (data not shown). Histopathologic examination of lungs at 4 weeks postinfection revealed that pulmonary lesions were more extensive in IL-36R−/− mice, which also contained a lower number of multinucleated giant cells compared with WT mice. Pulmonary lesions of IL-36R−/− also contained an increased number of inflammatory cells compared with WT mice (Figure 5F-H). The bacterial load in the lungs of IL-36R−/− mice tended to be higher than in WT mice; however, this difference was not statistically significant (Figure 5I). Taken together, these data support a role for endogenous IL-36 in enhancing Th1 immune responses protecting from BCG infection.
Critical role of IL-36β for the in vivo Th1 response to an intracellular bacterium. WT and IL-36R−/− mice were infected intravenously with 107 living M bovis BCG. On day 28, spleen cells of individual mice (n = 5 per genotype) were isolated, and cultured in the absence (Unstim) or presence of antigens derived from (A) M bovis BCG (BCG-Ag) or (B-D) viable M bovis BCG (103 CFU/well). Culture supernatants were harvested after 3 days of incubation and assayed by ELISA for (A) IFN-γ production, or by multiplex analysis for (B) IFN-γ, (C) IL-6, and (D) TNF-α production. (E) Accumulation of nitrite was measured using the Griess reagent. Values are the mean ± SD of 5 mice in each group. *P < .05 (Student t test), BCG-Ag, or BCG restimulation in WT cells significantly differs from the same stimulation in IL-36R−/− cells. Data are shown from one of 2 independent experiments with similar results. (F) Lung histopathologies at 4 weeks postinfection of IL-36R−/− (right panels) and WT (left panels) mice showing more extensive lesions in IL-36R−/− (original magnifications: ×40 top panel, and ×200 bottom panel). These results are representative of 2 independent experiments (n = 5 mice per group). (G) Evaluation of cellular content in lung sections from 4-week BCG-infected mice. Data are represented as the mean number of cells/field ± SD in 5 mice per group with at least 3 lobes analyzed per mouse. *P < .0005 (Student t test). (H) Determination of lung tissue/free space on lung sections. Data are represented as the mean of occupied/free space ± SD in 10 mice per group with at least 3 lobes analyzed per mouse. *P < .02 (Student t test). (I) Lung bacterial loads at 4 weeks postinfection are represented mean CFU per lung ± SD (n = 6-8 mice per group).
Critical role of IL-36β for the in vivo Th1 response to an intracellular bacterium. WT and IL-36R−/− mice were infected intravenously with 107 living M bovis BCG. On day 28, spleen cells of individual mice (n = 5 per genotype) were isolated, and cultured in the absence (Unstim) or presence of antigens derived from (A) M bovis BCG (BCG-Ag) or (B-D) viable M bovis BCG (103 CFU/well). Culture supernatants were harvested after 3 days of incubation and assayed by ELISA for (A) IFN-γ production, or by multiplex analysis for (B) IFN-γ, (C) IL-6, and (D) TNF-α production. (E) Accumulation of nitrite was measured using the Griess reagent. Values are the mean ± SD of 5 mice in each group. *P < .05 (Student t test), BCG-Ag, or BCG restimulation in WT cells significantly differs from the same stimulation in IL-36R−/− cells. Data are shown from one of 2 independent experiments with similar results. (F) Lung histopathologies at 4 weeks postinfection of IL-36R−/− (right panels) and WT (left panels) mice showing more extensive lesions in IL-36R−/− (original magnifications: ×40 top panel, and ×200 bottom panel). These results are representative of 2 independent experiments (n = 5 mice per group). (G) Evaluation of cellular content in lung sections from 4-week BCG-infected mice. Data are represented as the mean number of cells/field ± SD in 5 mice per group with at least 3 lobes analyzed per mouse. *P < .0005 (Student t test). (H) Determination of lung tissue/free space on lung sections. Data are represented as the mean of occupied/free space ± SD in 10 mice per group with at least 3 lobes analyzed per mouse. *P < .02 (Student t test). (I) Lung bacterial loads at 4 weeks postinfection are represented mean CFU per lung ± SD (n = 6-8 mice per group).
Discussion
Modulation of immune responses by the action of different cytokines is essential for optimal protection against invading microorganisms. Over the past decade, cytokines of the IL-1 family have been recognized for their crucial roles in innate and adaptive immune responses. These cytokines target distinct CD4+ T-cell subsets. IL-1 has a key role in the differentiation of Th17 cells, while IL-18 and IL-33 are known to enhance Th1 and Th2 polarization, respectively.10 In our previous study, we demonstrated that the newly named IL-36 cytokines exerted stimulatory effects on DCs and CD4+ T cells promoting Th1 responses in vitro and in vivo. This direct effect of IL-36 on immune cells was also demonstrated in human monocyte-derived DCs.33 Here, we show that, among T-cell subsets, IL-36R is mainly expressed on naive CD4+T cells and that IL-36 cytokines act directly on Th0 cells by enhancing cell expansion and IL-2 secretion, suggesting a pivotal role for IL-36 in the priming of immune responses. Moreover, we show that IL-36β acts as a copolarization factor with IL-12 to promote Th1 differentiation in vitro and is required for an efficient Th1 response to BCG infection in vivo.
We observed that IL-36R is essentially expressed on naive Th cells, while other IL-1R family members are predominantly expressed on other Th subsets. This finding suggests that IL-36 is able to promote early responses to infection within lymph nodes. Indeed, IL-36α and IL-36γ are expressed by BMDCs, whereas Th0 cells produce IL-36β. The endogenous expression of IL-36 in BMDCs and Th0 cells is in agreement with the existence of a positive autocrine and paracrine IL-36/IL-36R amplification loop, enhancing both IL-12 production by BMDCs, IL-2 secretion, and Th0 cell proliferation, and the effects of IL-12 and IL-18 on Th1 polarization (Figure 6).
Effects of IL-36 cytokines on the regulation of early Th1 polarization. On T cell–DC interaction by TCR/MHC engagement, IL-36R, which is constitutively expressed on both DCs and Th0 cells mediates cell activation by IL-36. On one hand, IL-36 produced by epithelial cells and DCs activates DCs to secrete IL-12, and on the other hand, IL-36 produced by Th0 cells activates Th0 cells, resulting in cell proliferation, survival of naive T cells, and IL-2 secretion. By a synergistic effect, IL-36 and IL-12 induce Th1 polarization in an IL-2–dependent manner through the induction of IL-12Rβ2 expression, leading to IFN-γ secretion, which is further amplified by the proliferative effect of IL-36 on Th0 cells. The formation of a positive feedback loop created by IL-36/IL-36R leads to sustained IFN-γ–mediated immune responses.
Effects of IL-36 cytokines on the regulation of early Th1 polarization. On T cell–DC interaction by TCR/MHC engagement, IL-36R, which is constitutively expressed on both DCs and Th0 cells mediates cell activation by IL-36. On one hand, IL-36 produced by epithelial cells and DCs activates DCs to secrete IL-12, and on the other hand, IL-36 produced by Th0 cells activates Th0 cells, resulting in cell proliferation, survival of naive T cells, and IL-2 secretion. By a synergistic effect, IL-36 and IL-12 induce Th1 polarization in an IL-2–dependent manner through the induction of IL-12Rβ2 expression, leading to IFN-γ secretion, which is further amplified by the proliferative effect of IL-36 on Th0 cells. The formation of a positive feedback loop created by IL-36/IL-36R leads to sustained IFN-γ–mediated immune responses.
In our coculture system, we showed that IL-36R signaling is necessary for polarization into IFN-γ–producing Th1 cells via IL-12 production and IL-2 secretion by BMDCs and Th0 cells, respectively. Of note, while the production of IFN-γ was abrogated in the absence of IL-12, IL-36 increased IL-4 production in cocultures of WT Th0 cells with IL-12p35−/− BMDCs (S.V., G.P., C.G., unpublished observations, April 2012), suggesting that, as previously described for IL-18 and IL-33, the selectivity of the effect of IL-36 on Th cell polarization is dependent on the cytokine environment. In agreement with these observations, we also observed that IL-36 up-regulated the expression of the Th2 transcription factor GATA-3 mRNA in Th0 cells and that this effect was inhibited by the addition of IL-12. In contrast to GATA-3, the expression of RORγt, the Th17 cell transcription factor, was significantly decreased in the presence of IL-36 alone or by combinations of IL-36 with IL-12 or IL-18 (S.V., G.P., C.G., unpublished observations, April 2012). Altogether, these observations are consistent with the observed effect of IL-36 on IL-2 production and the ability of IL-2 to induce Th1 and Th2 differentiation and to constrain Th17 generation.28
IL-2 is a pleiotropic cytokine with a broad array of actions including the ability to drive T-cell proliferation, influence cell survival, and promote Th1 differentiation.28,34-36 As IL-36 strongly induces IL-2 production, we hypothesized that the effects of IL-36 on T-cell proliferation and Th1 polarization might be dependent on IL-2. Our results show that IL-2 is totally required in IL-12 + IL-36β–induced Th1 differentiation by enhancing the expression of IL-12Rβ2. In contrast, IL-36 is able to enhance Th0 cell survival and proliferation independently of IL-2, but, as shown in IL-2−/− cells, IL-36 stimulatory effects are further enhanced in the presence of endogenous IL-2. Remarkably, suboptimal IL-2 neutralization abolished the effects of IL-36 on Th1 polarization, but not on cell survival and proliferation, thus indicating a differential sensitivity for IL-2 signaling in mediating the effect of IL-36 on T-cell proliferation, survival, and Th1 differentiation. Finally, we observed that the effect of IL-36β in enhancing IFN-γ production is both dependent on and independent of T-cell proliferation.
We demonstrated that IL-36R signaling is involved in Th1 immune responses in vivo using a systemic model of mycobacterial infection.37 Splenocytes of BCG-infected IL-36R−/− mice displayed impaired antigen-dependent IFN-γ responses compared with cells isolated from BCG-infected WT mice. Production of mediators involved in mycobacterial resistance, such as TNF-α, IL-6, or NO, was also impaired in IL-36R−/− splenocytes.38 Consistently, IL-36R−/− mice showed more severe lung pathology after BCG infection compared with WT mice, revealing an involvement of IL-36R signaling in host protection against intracellular bacterial infection. In view of previously described protective roles of IL-18 and IL-12 during mycobacterial infection in vivo,39-41 the study of potential synergies or redundancies of IL-36 with these Th1 cytokines will be of great interest. Collectively, our findings have demonstrated that IL-36 acts as an early danger signal that modulates immune responses, via IL-12 and IL-2 signaling. Moreover, the present study describes for the first time a protective role of endogenous IL-36 in the host response to mycobacterial infection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Caroline Pot (Division of Neurology, University Hospitals of Geneva) for helpful discussions.
This work was supported by Swiss National Foundation grants 310030-135195 (C.G.) and 310030-134691 (G.P.), the Rheuma-search Foundation, and the Institute of Arthritis Research. The salary of S.V. was supported by a postdoctoral fellowship grant from the Novartis Foundation.
Authorship
Contribution: S.V. planned studies, performed experiments, analyzed data, and wrote the manuscript; G.P. analyzed data and wrote the manuscript; P.M., C.L., D.S., E.R., M.L.O., D.V., and F.R. performed experiments and analyzed data; I.G., F.S., and J.E.S. analyzed data and wrote the manuscript; and C.G. supervised the project, planned studies, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: J.E.S. was an employee of Amgen and owns Amgen stocks and stock options. The remaining authors declare no competing financial interests.
The current affiliation for J.E.S. is Department of Pathology-Immunology, University of Geneva School of Medicine, Geneva, Switzerland.
Correspondence: Cem Gabay, MD, Division of Rheumatology, University Hospitals of Geneva, 26 Avenue Beau-Séjour, 1211 Geneva 14, Switzerland; e-mail: cem.gabay@hcuge.ch.

![Figure 1. IL-36R is highly expressed and functional in naive CD4+ T cells. CD44loCD62Lhi naive CD4+ T cells (Th0 cells) were FACS sorted and polarized or not under Th1, Th2, or Th17 cell conditions for 5 days. Total mRNA was isolated from naive Th cells directly after the sort and from Th1, Th2, and Th17 differentiated cells for analyses by quantitative RT-PCR. Results represent (A) IL-36R, (B) IL-1R1, (C) IL-18Rα, and (D) ST2 mRNA expression levels relative to GAPDH. Error bars represent the SD of the mean of 3 independent experiments. (E-F) Naive T cells from WT and IL-36R−/− mice (4 × 104 cells per well) were sorted and cultured in 96-well plates precoated with anti-CD3 and anti-CD28 mAb and incubated for 72 hours in the absence (Medium) or presence of IL-36R ligands, IL-1β, IL-33, IL-18, IL-12, or LPS (100 ng/mL). (E) IL-2 secretion in culture supernatants was determined by ELISA. (F) Proliferative responses were assessed by [3H]-thymidine incorporation. Data are shown from one of 3 independent experiments with similar results. Error bars represent the SD of triplicates in the same experiment. *P < .05 (Student t test); IL-36R agonist stimulation significantly differs from unstimulated cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/17/10.1182_blood-2012-06-439026/4/m_zh89991298550001.jpeg?Expires=1769081655&Signature=jtVbFnKHyrKozbAwcmkizawNllqoUnjChWMLxZcqbDA2OPI9eDLxup7z2ileqou81h8IIpLOSsXhZhIA~5ZQ9WfxF7cXxkUlRW1f9GflvaugxbJqCd4cSTHbqOzXH~8mNQHNz0ZX8tW59fZ8hxtvpgMt~YpYY1qEJERKkZiec6Xas8cNTXFNbJodcOGwFGz9LZFyG5QGkcBkYuQSQxZe0uSLnN0sy64ARQgpQWHwOfRHm0LM4uQcePbJneM0rQY8l-DVX6Ig-X1rG3T~bkaAoqEbHVqSV0llYO0EYmuXLXLKIx5-oDg7d0d7LTfw6OYdqb8c7f0Nx5QwzmYV-ICG8w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal