Abstract
Despite the potent immunosuppressive activity that mesenchymal stem cells (MSCs) display in vitro, recent clinical trial results are disappointing, suggesting that MSC viability and/or function are greatly reduced after infusion. In this report, we demonstrated that human MSCs activated complement of the innate immunity after their contact with serum. Although all 3 known intrinsic cell-surface complement regulators were present on MSCs, activated complement overwhelmed the protection of these regulators and resulted in MSCs cytotoxicity and dysfunction. In addition, autologous MSCs suffered less cellular injury than allogeneic MSCs after contacting serum. All 3 complement activation pathways were involved in generating the membrane attack complex to directly injure MSCs. Supplementing an exogenous complement inhibitor, or up-regulating MSC expression levels of CD55, one of the cell-surface complement regulators, helped to reduce the serum-induced MSC cytotoxicity. Finally, adoptively transferred MSCs in complement deficient mice or complement-depleted mice showed reduced cellular injury in vivo compared with those in wild type mice. These results indicate that complement is integrally involved in recognizing and injuring MSCs after their infusion, suggesting that autologous MSCs may have ad-vantages over allogeneic MSCs, and that inhibiting complement activation could be a novel strategy to improve existing MSC-based therapies.
Introduction
Mesenchymal stem cells (MSCs) are adult stem cells that can differentiate into many types of cells, and they are strongly immunosuppressive.1 MSCs have great potential in inflammatory disease treatment and in regenerative medicine.2 There have been approximately 150 MSC-based clinical trials registered in the www.clinicaltrials.gov website alone for the treatment of different diseases, including graft-versus-host disease (GVHD), Crohn disease, diabetes, multiple sclerosis (MS), myocardial infarction, and allograft rejection. It is widely believed in the field that MSCs are able to escape from the host immune surveillance; therefore, MSCs expanded from 1 donor can be used to treat other patients without being rejected.2,3 However, despite their apparent immunosuppressive activity in vitro, results from recent MSCs clinical trials were not very encouraging.4 One possible explanation for these disappointing results is that immediately after systemic infusion, these MSCs are recognized and injured by the host, leading to severely reduced activity in vivo.
Complement is an important part of the innate immunity, which serves as the first shield against anything foreign, and complement components are abundant in the serum.5 Complement can be activated through 3 different pathways: the classic, the alternative, and the lectin pathway. Activated complement recruits and activates leukocytes, enhances phagocytosis, and forms membrane attack complex (MAC) to directly injure the target cells. It has been established that complement plays an important role in the rejection of transplanted xeno/allografts,6,7 and complement inhibitors are being developed as new drugs to prevent xeno/allograft rejection. However, the potential roles of complement in recognizing in vitro expanded autologous (auto)/allogeneic (allo) MSCs after their infusion, and the detailed underlying mechanisms are unclear.
Methods
MSCs and other reagents
Primary bone marrow-derived human MSCs from healthy donors were provided by the MSC Core Facility at the National Center for Regenerative Medicine (NCRM) of Case Western Reserve University,8-10 and by Tulane University Center of Gene Therapy.11 Sera from the same donors were also collected at the time of bone marrow aspiration. MSCs were further characterized by analyzing the expression profiles of cell surface markers (CD34, CD45, CD73, and CD105), and by testing their plasticity of differentiating into adipocytes and osteoblasts (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). MSCs from Tulane University were cultured in α-MEM with 16% FBS (Invitrogen), and MSCs from NCRM were cultured in DMEM with 10% FBS (Invitrogen), following the provided protocols. MSCs with passage numbers 3 to 6 were used in all experiments. Normal human sera (NHS) and different complement component-depleted human sera (C3-dpl, C1q-dpl, factor B-dpl, and C4-dpl sera) were ordered from Complement Tech. Cobra venom factor (CVF) was purchased from Sigma-Aldruch. Mannose-binding lectin (MBL)–depleted human sera were prepared as previously described.12 Human peripheral blood mononuclear cells (PBMCs) were provided by the Hematopoietic Stem Cell Core Facility at Case Western Reserve University. Wild-type (WT) and C3−/− mice (all C57BL/6 mice) were ordered from Jackson Laboratory. All animal studies were conducted following an approved Institutional Animal Care Protocol. Human MSCs and PBMC isolation procedures were approved by the Institutional Review Boards (IRB) of Case Western Reserve University.
C3 deposition assay
MSCs (1 × 106) were incubated at 37°C for 30 minutes with 30 μL of NHS in 100 μL gelatin veronal buffer++ (GVB++) with (control) or without 10μM of EDTA. After this, cells were spun down and washed with 1 mL of FACS buffer (PBS plus 1% BSA) twice, then incubated with 5 μg/mL of FITC-labeled rat anti–mouse C3 IgG (Cedarlane). After washing, cells were analyzed by flow cytometry (LSR I; BD Bioscience).
In vitro cytotoxicity assay
MSC cytotoxicity after incubation with serum was assessed by a BCECF [2′, 7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein] leakage assay.13 In brief, 2 × 105 MSCs were first labeled by incubation with 5μM of BCECF-AM (Invitrogen) for 30 minutes at 37°C. After washing, the labeled MSCs were incubated with different amounts of NHS or respective complement-depleted sera in 100 μL of GVB++ buffer for another 30 minutes at 37°C. After this, supernatants were harvested, and the released BCECF was measured by a fluorescence microtiter plate reader (Molecular Devices) with excitation and emission wavelengths of 485 nm and 538 nm. To calculate the percentage of BCECF release (complement mediated injury), the following equation was used as reported before14,15 : percentage of BCECF release (cytotoxicity) = [(A−B)/(C−B)] ×100%; where A represents the mean experimental BCECF release, B represents the mean spontaneous BCECF release, and C represents the mean maximum BCECF released that was induced by incubating cells with 0.5% SDS.
In vivo cytotoxicity assay
To verify the above in vitro results, a similar BCECF leakage assay was used in vivo. In brief, MSCs were in vitro labeled with 10μM of BCECF at 37°C for 30 minutes, then the same numbers of the labeled-MSCs (1.0 × 106/mouse) were adoptively transferred into WT and complement deficient (C3−/−) mice by intravenous tail vein injection. Mice were bled every 20 minutes from the tail after the infusion, and the leaked BCECF in the serum were measured by a fluorescence microtiter plate reader (Molecular Devices) with excitation and emission wavelengths of 490 nm and 535 nm after 1:50 dilution in PBS buffer. Similar in vivo cytotoxicity assays were also carried out with complement-depleted mice. To deplete complement in mice in vivo, 20 μg of CVF were injected into each of the WT mice intraperitoneally following protocols established previously in the laboratory,13 and the BCECF-labeled MSCs were infused 60 minutes later.
Sera IgG depletion (reduction)
To deplete (reduce) IgGs in the sera, 100 μL of NHS were incubated with 20 μL of protein A-magnetic beads (Millipore) for 30 minutes at room temperature with rotation, then spun at 2000 rpm for 5 minutes. The supernatants were collected as IgG-depleted (reduced) sera for MSC cytotoxicity assays.
MSC function assay
The impact of activated complement on MSC immunosuppressive function was assessed by a carboxyfluorescein succinimidyl ester (CFSE)–based T-cell proliferation assay. In brief, MSCs were incubated with or without 30% NHS in GVB++ buffer for 30 minutes. After washing, identical numbers of MSCs were then cocultured with CFSE-labeled, activated human T cells. In 48 hours, the efficacies of different MSCs in inhibiting T-cell proliferation were assessed by flow cytometry, gating on CD4+ cells. For human T-cell activation, PBMCs were incubated with anti-CD3/CD28 Dynabeads (Invitrogen) at a ratio of 1:1 following manufacturer provided protocols.
Cell surface complement inhibitor assays
For intrinsic cell-surface complement regulator detections, 2 × 105 MSCs were incubated with 5 μg/mL of mouse anti-CD55 (Clone JS11; Biolegend), anti-CD46 (Clone MEM-258; Biolegend), anti-CD59 IgG (Clone H19; Biolegend) or isotype controls in 100 μL of PBS buffer with 5% BSA for 30 minutes on ice. After washing, the cells were incubated with 2 μg/mL of Alexa 488–conjugated goat anti–mouse IgG (Sigma-Aldrich), followed by flow cytometry analysis (LSR I; BD Bioscience). To examine the role of respective cell-surface complement inhibitors in protecting allogeneic MSCs from complement-mediated injury, MSCs were incubated with 10 μg/mL of the previously mentioned IgGs against CD46, CD55, or CD59 to block their function. After washing, the cells with different blocked cell-surface complement inhibitors were tested in a conventional complement-mediated cytotoxicity assay.
Up-regulation of CD55 expression levels on MSCs
To up-regulate CD55 expression levels on MSCs, a human CD55-expressing, and a mouse CD55 (Daf1)–expressing recombinant adenovirus with a green fluorescence protein (GFP) marker were constructed using an AdEasy kit (Stratagene) following protocols provided by the manufacturer. The titer of the adenovirus was determined by counting green transfected cells under a fluorescence microscope (Eclipse TS100; Nikon). For in vitro cytotoxicity assays with NHS, MSCs were incubated with the human CD55-expressing adenovirus or empty adenovirus (MOI: 1:10) for 48 hours, then the human CD55 levels on cell surface were assessed by flow cytometry using mouse anti–human CD55 IgG (clone 2H6; BD Bioscience) and APC-labeled goat anti–mouse IgG (Invitrogen). After this, these cells were also analyzed by the same BCECF-leakage assays to measure their susceptibility to complement-mediated attack. For in vivo cytotoxicity assays in WT mice, MSCs were transfected with the mouse CD55–expressing adenovirus or the control virus, and infused into WT mice (1 × 106/mouse by intravenous injection) for the BCECF-leakage based cytotoxicity assays.
Inhibiting MAC assembly by an anti-C5 IgG
To assess the efficacy of inhibiting the formation of MAC in protecting MSCs from complement-mediated attack, MSCs with 10, 20, and 40 μg/mL of a goat anti-C5 IgG or control goat IgG (Cedarlane) were analyzed in the same BCECF-based complement-mediated cytotoxicity assays.
Results
MSCs are recognized and injured by complement after their contact with serum
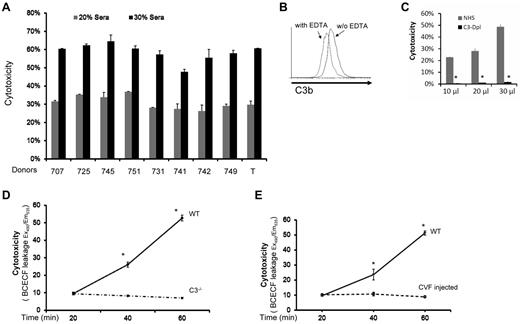
We first studied MSCs from 9 healthy donors in a conventional BCECF-based cytotoxicity assay16 after incubating them with nonrelated normal human serum (NHS). We found that all MSCs had significant amounts of BCECF leakage after their contact with serum (in a dose-dependent manner), indicating that these MSCs were injured by the serum after incubation (Figure 1A). To determine whether complement was activated during the MSC-serum interactions, we incubated 1 of the MSCs (T) with NHS, and assessed the complement activation product C3b on the cell surface by flow cytometry. These assays showed that C3b was detectable on the surface of MSCs after incubation, indicating that complement was activated when MSCs contact serum (Figure 1B). Next, we repeated the cytotoxicity assays in which the MSCs were incubated with 10 μL, 20 μL, and 30 μL of NHS, or the same amounts of C3-depleted sera. These assays showed that the MSCs incubated with NHS were injured in a dose-dependent manner as discovered before (Figure 1C), whereas the MSCs incubated with the same amount of C3-depleted sera (no complement activation) only had minimal leakage of BCECF (Figure 1C), indicating that MSCs were injured by complement after their contact with serum.
MSCs are injured by complement after their contact with serum. (A) MSCs are injured after incubation with serum. Five × 105 of MSCs from different health donors (707, 725, 745, 751,731,741,742,749, and T) were first labeled with BCECF, then incubated with 20μL and 30 μL NHS in 100 μL of GVB++ buffer at 37°C for 30 minutes. MSCs cytotoxicity was assessed by measuring BCECF leaked into the supernatants. Representative results of 3 individual experiments. Data are mean ± SD, *P < .05. (B) Complement activation product C3b detection on MSC cel-surface. MSCs (1 ×106) were incubated with 30 μL of NHS in 100 μL of GVB++ buffer either without or with 1μM of EDTA (to inhibit complement activation). After incubation at 37°C for 30 minutes, the washed cells were stained with 5 μg/mL of FITC-labeled anti–human C3b mAb, and analyzed by a flow cytometer (LSR II). Representative results of 3 individual experiments. (C) Role of complement in the serum-mediated MSCs cytotoxicity (in vitro). Five × 105 of BCECF-labeled MSCs were incubated with 10, 20, or 30 μL of NHS or C3–depleted sera (C3-Depl) in 100 μL of GVB++ buffer. After incubation at 37°C for 30 minutes, MSCs cytotoxicity was assessed by measuring BCECF leaked into the supernatants. Representative results of 3 individual experiments. Data are mean ± SD, *P < .05. (D-E) Role of complement in serum-mediated MSCs cytotoxicity after infusion (in vivo). 1.0 × 106 BCECF-labeled MSCs were adoptively transferred into WT, C3−/− (D) and complement-depleted mice (E) by tail vein intravenous injection, serum levels of leaked BCECF in these mice were measured 20, 40, and 60 minutes after the injection. n = 3 in each group, data are mean ± SD, *P < .001.
MSCs are injured by complement after their contact with serum. (A) MSCs are injured after incubation with serum. Five × 105 of MSCs from different health donors (707, 725, 745, 751,731,741,742,749, and T) were first labeled with BCECF, then incubated with 20μL and 30 μL NHS in 100 μL of GVB++ buffer at 37°C for 30 minutes. MSCs cytotoxicity was assessed by measuring BCECF leaked into the supernatants. Representative results of 3 individual experiments. Data are mean ± SD, *P < .05. (B) Complement activation product C3b detection on MSC cel-surface. MSCs (1 ×106) were incubated with 30 μL of NHS in 100 μL of GVB++ buffer either without or with 1μM of EDTA (to inhibit complement activation). After incubation at 37°C for 30 minutes, the washed cells were stained with 5 μg/mL of FITC-labeled anti–human C3b mAb, and analyzed by a flow cytometer (LSR II). Representative results of 3 individual experiments. (C) Role of complement in the serum-mediated MSCs cytotoxicity (in vitro). Five × 105 of BCECF-labeled MSCs were incubated with 10, 20, or 30 μL of NHS or C3–depleted sera (C3-Depl) in 100 μL of GVB++ buffer. After incubation at 37°C for 30 minutes, MSCs cytotoxicity was assessed by measuring BCECF leaked into the supernatants. Representative results of 3 individual experiments. Data are mean ± SD, *P < .05. (D-E) Role of complement in serum-mediated MSCs cytotoxicity after infusion (in vivo). 1.0 × 106 BCECF-labeled MSCs were adoptively transferred into WT, C3−/− (D) and complement-depleted mice (E) by tail vein intravenous injection, serum levels of leaked BCECF in these mice were measured 20, 40, and 60 minutes after the injection. n = 3 in each group, data are mean ± SD, *P < .001.
Because human MSCs have been extensively tested in mouse models, we chose to verify these in vitro results in vivo using WT and C3–deficient mice. We first tested whether human MSCs can be injured by WT mouse serum through complement. We found that mouse C3b deposition was detectable on human MSCs cell-surface after incubation with WT mouse serum, and that these MSCs were injured by mouse serum in a dose-dependent manner (not shown). We then adoptively transferred BCECF-labeled MSCs into WT and complement-deficient mice (C3−/− mice), and measured leaked BCECF levels in the serum at different time points. These assays showed that, compared with time-dependent increase of leaked BCECF levels in the WT mice, BCECF leakage in the complement-deficient mice remained almost flat at different time points (Figure 1D). Adoptive transfer of the BCECF-labeled MSCs into WT and complement-depleted mice (by CVF injection) showed the same pattern as discovered in C3−/− mice (Figure 1E). These results indicated that similar to what was discovered in the in vitro studies, MSCs were attacked and injured immediately after infusion, and that complement was integrally involved in the underlying mechanism.
Auto-MSCs suffer less cellular injury than allo-MSCs after contact with serum
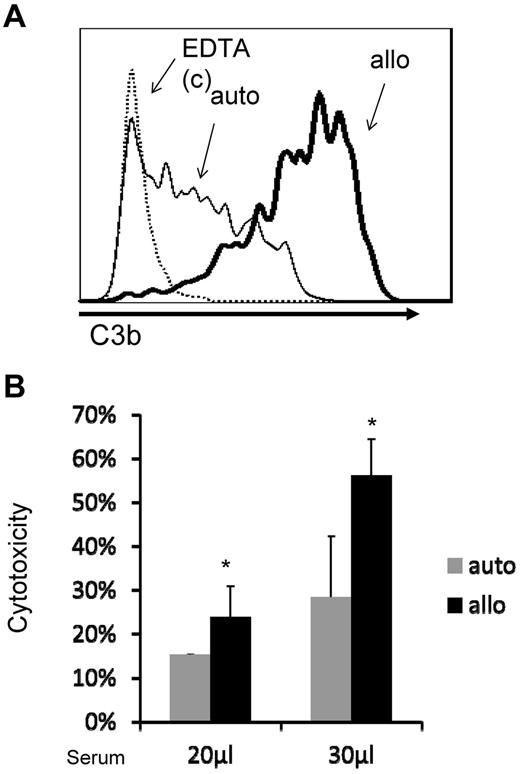
It is unclear whether auto-MSCs could have any advantages over allo-MSCs after infusion. To address this, we incubated sera with MSCs from the same donor (auto), and MSCs from a different donor (allo), and then compared levels of complement activation (C3b deposition) on the cell surface by flow cytometry. We also compared serum-mediated cytotoxicity between the auto and allo-MSCs after their incubation with serum. These studies found that even auto-MSCs activated complement after their contact with serum. However, they provoked significantly less complement activation than the allo-MSCs as indicated by the markedly reduced levels of C3b deposition on the cell surface shown by flow cytometry (Figure 2A). Consistent with these data, cytotoxicity assays showed that auto-MSCs had significantly less BCECF leakage than allo-MSCs after incubation with the serum (Figure 2B), suggesting that auto-MSCs could have advantages over allo-MSCs in clinic by not provoking intense complement activation after infusion.
Auto-MSCs suffer less cellular injury than allo-MSCs after contacting with serum. Serum from donor 741 were incubated with MSCs from donor 741(auto) and donor 731(allo) at 37°C for 30 minutes. C3 deposition on the cell surface (incubated with 30 μL of serum) was assessed by flow cytometry (A), and MSCs cytotoxicity was assessed by measuring levels of BCECF leaked into the culture supernatants (B). Representative results of 4 serum incubated with matched and unmatched MSCs experiments.
Auto-MSCs suffer less cellular injury than allo-MSCs after contacting with serum. Serum from donor 741 were incubated with MSCs from donor 741(auto) and donor 731(allo) at 37°C for 30 minutes. C3 deposition on the cell surface (incubated with 30 μL of serum) was assessed by flow cytometry (A), and MSCs cytotoxicity was assessed by measuring levels of BCECF leaked into the culture supernatants (B). Representative results of 4 serum incubated with matched and unmatched MSCs experiments.
Serum-incubated MSCs are less potent in suppressing T-cell responses
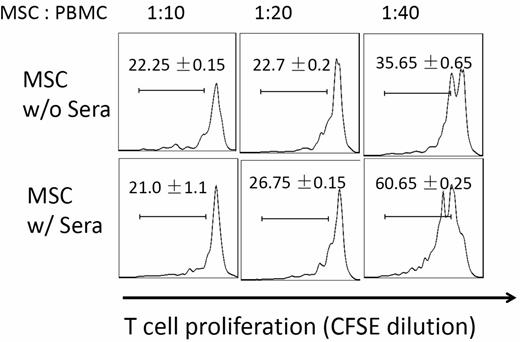
Many of the ongoing clinical trials are based on the observation that MSCs can potently inhibit T-cell responses. We next tested whether MSC function was compromised after their contact with serum by comparing potencies of MSCs with or without serum incubation in suppressing activated T-cell proliferation. We first labeled human PBMCs with CFSE, and activated the T cells with anti-CD3/CD28 Dynabeads (Invitrogen). After the T-cell activation, we added the same numbers of MSCs incubated with NHS or PBS into the cultures. After 96 hours of coculture, we assessed MSCs T-cell inhibition efficacy by measuring T-cell proliferation based on CFSE dilution. These experiments (Figure 3) showed that at the ratios of 1:20 and 1:40 (MSCs: T cells), T-cell proliferation was stronger in wells where MSCs with serum incubation were added than in wells where MSCs without serum incubation were added, indicating that MSCs had reduced potency in inhibiting activated T-cell proliferation after their contact with serum.
Complement-injured MSCs are less potent in suppressing T-cell responses. MSCs (2 × 106) were incubated at 37°C with or without 30 μL of NHS in 100 μL of GVB++ buffer for 30 minutes. After this, washed MSCs were mixed with CFSE-labeled, and activated human T cells at different ratios. For human T-cell activation, 1 × 106 of PBMCs were incubated with anti-CD3/CD28 Dynabeads following manufacturer provided protocols. After 4 days of incubation at 37°C, the efficacy of MSCs in inhibiting activated T-cell proliferation was assessed by measuring CFSE dilution on CD4+ T cells using flow cytometry. Representative results of 3 individual experiments. Data are mean ± SD
Complement-injured MSCs are less potent in suppressing T-cell responses. MSCs (2 × 106) were incubated at 37°C with or without 30 μL of NHS in 100 μL of GVB++ buffer for 30 minutes. After this, washed MSCs were mixed with CFSE-labeled, and activated human T cells at different ratios. For human T-cell activation, 1 × 106 of PBMCs were incubated with anti-CD3/CD28 Dynabeads following manufacturer provided protocols. After 4 days of incubation at 37°C, the efficacy of MSCs in inhibiting activated T-cell proliferation was assessed by measuring CFSE dilution on CD4+ T cells using flow cytometry. Representative results of 3 individual experiments. Data are mean ± SD
All complement activation pathways are involved in injuring MSCs
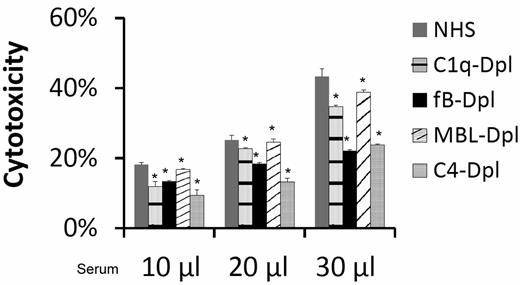
To elucidate the mechanism by which complement is activated to injure MSCs, we incubated MSCs with the same amount of NHS, C1q-depleted sera (classic pathway-deficient), factor B-depleted sera (alternative pathway-deficient), MBL-depleted (lectin pathway-deficient), or C4-depleted (both classic and lectin pathway-deficient) sera, and performed the BCECF-based cytotoxicity assay again. These studies (Figure 4) found that incubation of MSCs with the complement activation pathway-deficient sera resulted in only partially reduced BCECF leakage, indicating that all of the complement activation pathways are involved nonexclusively in the serum mediated MSC cytotoxicity.
All complement activation pathways are involved in serum-mediated MSC cytotoxicity. MSCs (5 × 105) were subjected to the same BCECF leakage-based cytotoxicity assays with 10, 20, and 30 μL of NHS, C1q-depleted sera (classic pathway-deficient), fB-depleted sera (alternative pathway-deficient), MBL-depleted (lectin pathway-deficient) or C4-depleted sera (both classic and lectin pathways deficient) in 100 μL of GVB++ buffer. After incubation at 37°C for 30 minutes, MSCs cytotoxicity was assessed by measuring BCECF leaked into the supernatants. Representative results of 3 individual experiments. Data are mean ± SD, *P < .05;
All complement activation pathways are involved in serum-mediated MSC cytotoxicity. MSCs (5 × 105) were subjected to the same BCECF leakage-based cytotoxicity assays with 10, 20, and 30 μL of NHS, C1q-depleted sera (classic pathway-deficient), fB-depleted sera (alternative pathway-deficient), MBL-depleted (lectin pathway-deficient) or C4-depleted sera (both classic and lectin pathways deficient) in 100 μL of GVB++ buffer. After incubation at 37°C for 30 minutes, MSCs cytotoxicity was assessed by measuring BCECF leaked into the supernatants. Representative results of 3 individual experiments. Data are mean ± SD, *P < .05;
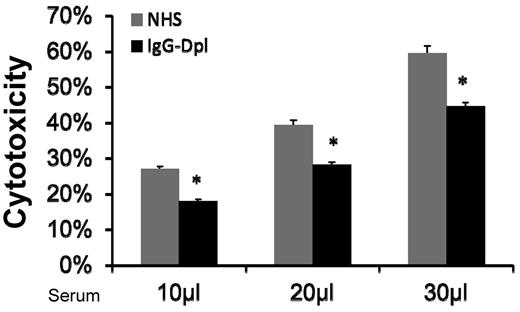
Depletion (reduction) of IgGs in the serum reduced MSC cytotoxicity
Natural antibodies are reported in allo/xeno transplantation studies.17,18 One possible mechanism by which MSCs are injured by the activated complement in serum is that naturally existing IgGs bind to MSC cell-surface, and initiate complement activation through the classic pathway. If this is true, then depleting (reducing) IgG levels in the NHS should reduce serum-induced MSC cytotoxicity. To test this, we incubated NHS with protein A-beads, and then depleted (reduced) serum IgGs by spinning the beads down and collecting the supernatants. ELISA assays using antibodies specific for human IgGs showed that majority of the IgGs in the serum were depleted after these procedures (supplemental Figure 2). We then compared MSC cytotoxicity after incubating them with NHS or NHS treated with the protein A-beads. We found that depleting (reducing) IgG in the sera using protein A-beads decreased, but did not abolish, the serum-mediated MSCs cytotoxicity (Figure 5). This data are also consistent with the results using classic pathway-deficient sera, which showed moderate reduction, but not complete abolishment of complement-mediated MSC injury. Taken together, these results suggest that one of the mechanisms by which MSCs are injured after infusion is that after their contact with serum, naturally existing MSC-reactive antibodies in the serum bind to the MSCs, then activate complement through the classic pathway, leading to MSC cytotoxicity.
Depletion (reduction) of serum IgGs moderately reduces MSC cytotoxicity. IgGs in NHS were depleted (reduced) using protein A beads. After this, MSCs were cultured with 10, 20, and 30 μL of NHS or IgG-depleted NHS in 100 μL of GVB++ buffer. After incubation at 37°C for 30 minutes, MSC cytotoxicity was assessed by measuring levels of BCECF leaked into the supernatants. Representative results of 3 individual experiments. Data are mean ± SD, *P < .05;
Depletion (reduction) of serum IgGs moderately reduces MSC cytotoxicity. IgGs in NHS were depleted (reduced) using protein A beads. After this, MSCs were cultured with 10, 20, and 30 μL of NHS or IgG-depleted NHS in 100 μL of GVB++ buffer. After incubation at 37°C for 30 minutes, MSC cytotoxicity was assessed by measuring levels of BCECF leaked into the supernatants. Representative results of 3 individual experiments. Data are mean ± SD, *P < .05;
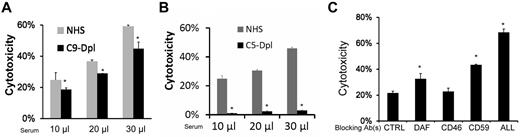
MSC cytotoxicity is mediated through MAC
Formation of MAC is a major mechanism by which activated complement attacks target cells.19 To determine the role of MAC in the newly discovered complement-mediated injury of MSCs, we first compared cytotoxicity of MSCs incubated with NHS, or C9–depleted sera, which lacks the C9 that is required to form the complete C5b-9 MAC complex. These assays showed that although C9–depleted sera cannot form complete MAC, MSCs incubated in these sera had only mildly reduced BCECF leakage compared with those incubated in NHS (Figure 6A). We next repeated the cytotoxicity assays using NHS and C5-depleted sera, in which the formation of MAC cannot be initiated. These experiments found that incubating MSCs with the C5-depleted sera led to little, if any, BCECF leakage (Figure 6B). These data, taken together, indicate that MAC plays a primary role in the complement-mediated MSC cytotoxicity, and that the partially assembled C5b-8 complexes are enough to cause cellular injury.
MSCs cytotoxicity is mediated through MAC. MSCs (5 × 105) were subjected to the same BCECF leakage-based cytotoxicity assays with 10, 20, and 30 μL of NHS, C9–depleted (A) or C5–depleted sera (B) in 100 μL of GVB++ buffer. After incubation at 37°C for 30 minutes, MSCs cytotoxicity was assessed by measuring BCECF leaked into the supernatants. Representative results of 3 individual experiments. Data are mean ± SD, *P < .05. (C) Blocking the assembly of MAC reduces MSC cytotoxicity after their contact with sera. MSCs (5 × 105) were cultured with 30 μL of NHS in 100 μL of GVB++ buffer. After the setup, the cells were divided into 2 groups. Ten, 20, or 40 μg/mL of goat anti-C5 IgG or control goat IgG were then added. After incubation at 37°C for 30 minutes, MSC cytotoxicity was assessed by measuring levels of BCECF leaked into the supernatants. Representative results of 3 individual experiments. Data are mean ± SD, *P < .05.
MSCs cytotoxicity is mediated through MAC. MSCs (5 × 105) were subjected to the same BCECF leakage-based cytotoxicity assays with 10, 20, and 30 μL of NHS, C9–depleted (A) or C5–depleted sera (B) in 100 μL of GVB++ buffer. After incubation at 37°C for 30 minutes, MSCs cytotoxicity was assessed by measuring BCECF leaked into the supernatants. Representative results of 3 individual experiments. Data are mean ± SD, *P < .05. (C) Blocking the assembly of MAC reduces MSC cytotoxicity after their contact with sera. MSCs (5 × 105) were cultured with 30 μL of NHS in 100 μL of GVB++ buffer. After the setup, the cells were divided into 2 groups. Ten, 20, or 40 μg/mL of goat anti-C5 IgG or control goat IgG were then added. After incubation at 37°C for 30 minutes, MSC cytotoxicity was assessed by measuring levels of BCECF leaked into the supernatants. Representative results of 3 individual experiments. Data are mean ± SD, *P < .05.
Blocking the assembly of MAC by anti-C5 IgGs reduces MSCs cytotoxicity after their contact with serum
These results suggested that blocking C5 should be effective to reduce MSC cytotoxicity after their contact with serum. To test this, we repeated the BCECF-based cytotoxicity assay, and this time, we added different concentrations of an anti–human C5 IgG or control IgG into the cultures to inhibit MAC assembly. These assays (Figure 6C) showed that adding anti-C5 IgG significantly reduced MSCs cytotoxicity after their contact with sera, indicating that inhibition of MAC formation by supplementing exogenous complement inhibitors like the anti-C5 IgGs will be effective in protecting MSCs from serum-mediated cytotoxicity after infusion.
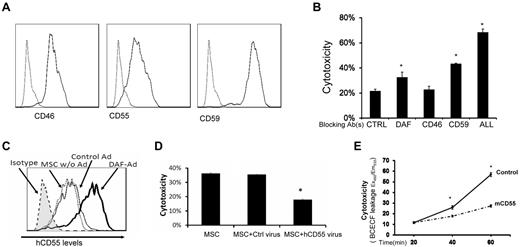
MSCs constitutively express all of the intrinsic cell-surface complement regulators
Intrinsic cell-surface complement regulators are critical in protecting cells from complement-mediated attack.20 We next examined the distribution of all known intrinsic cell surface complement inhibitors CD55,21 CD59,22 and CD4623 on MSCs by flow cytometry. These assays demonstrated that all 3 intrinsic cell-surface complement regulators were present on MSCs (Figure 7A). To determine the respective roles of these complement regulators in protecting MSCs from complement-mediated attack, we blocked the complement regulators' function either individually or in combination using their respective neutralizing monoclonal antibodies (mAbs), then subjected the blocked MSCs to the same BCECF-based cytotoxicity assays. These assays (Figure 7B) showed that blocking either CD55 or CD59 made the MSCs significantly more susceptible to serum-mediated injury, whereas blocking CD46 alone had no significant effect. In addition, blocking all 3 intrinsic cell-surface complement regulators on the MSCs led to markedly increased cytotoxicity after serum incubation.
Roles of intrinsic cell surface complement regulators in protecting MSCs after their contact with serum. (A) MSCs constitutively express all of the intrinsic cell-surface complement regulators. MSCs (5 × 105) were stained with 5 μg/mL of anti-CD55 IgG, anti-CD46 IgG, anti-CD59 IgG (solid lines), or isotype controls (dotted line) for 30 minutes on ice, then analyzed by flow cytometry. (B) Respective protective roles of these complement regulators. MSCs were incubated 10 μg/mL of control IgG, anti-CD55 IgG, anti-CD46 IgG, anti-CD59 IgG, or all of the IgGs, then subjected to the same BCECF leakage-based cytotoxicity assays. Representative results of 3 individual experiments. Data are mean ± SD, *P < .05. (C-D) Up-regulating human CD55 expression reduces MSC cytotoxicity after their contact with human serum in vitro. Eighty percent confluent MSCs in a well of a 6-well plate were transfected with CD55-expressing adenovirus or the same titer of empty control virus. In 48 hours, transfection efficiency was monitored by checking GFP expression under a fluorescence microscope (not shown), and levels of CD55 on MSCs cell surface was assessed by flow cytometry. (C) CD55 levels on different MSCs. Shaded area, isotype control; dotted line, MSCs without transfection; thin line, MSCs transfected with control virus; thick line, MSCs transfected with CD55-expressing virus. (D) Five × 105 of each MSCs without transfection, MSCs transfected with control virus, and MSCs transfected with CD55-expressing virus were subjected to the same BCECF leakage-based cytotoxicity assays. (E) Up-regulating mouse CD55 expression reduces MSCs cytotoxicity in vivo after infusion (n = 3 in each group, *P < .05). Representative results of 3 individual experiments. Data are mean ± SD, *P < .05.
Roles of intrinsic cell surface complement regulators in protecting MSCs after their contact with serum. (A) MSCs constitutively express all of the intrinsic cell-surface complement regulators. MSCs (5 × 105) were stained with 5 μg/mL of anti-CD55 IgG, anti-CD46 IgG, anti-CD59 IgG (solid lines), or isotype controls (dotted line) for 30 minutes on ice, then analyzed by flow cytometry. (B) Respective protective roles of these complement regulators. MSCs were incubated 10 μg/mL of control IgG, anti-CD55 IgG, anti-CD46 IgG, anti-CD59 IgG, or all of the IgGs, then subjected to the same BCECF leakage-based cytotoxicity assays. Representative results of 3 individual experiments. Data are mean ± SD, *P < .05. (C-D) Up-regulating human CD55 expression reduces MSC cytotoxicity after their contact with human serum in vitro. Eighty percent confluent MSCs in a well of a 6-well plate were transfected with CD55-expressing adenovirus or the same titer of empty control virus. In 48 hours, transfection efficiency was monitored by checking GFP expression under a fluorescence microscope (not shown), and levels of CD55 on MSCs cell surface was assessed by flow cytometry. (C) CD55 levels on different MSCs. Shaded area, isotype control; dotted line, MSCs without transfection; thin line, MSCs transfected with control virus; thick line, MSCs transfected with CD55-expressing virus. (D) Five × 105 of each MSCs without transfection, MSCs transfected with control virus, and MSCs transfected with CD55-expressing virus were subjected to the same BCECF leakage-based cytotoxicity assays. (E) Up-regulating mouse CD55 expression reduces MSCs cytotoxicity in vivo after infusion (n = 3 in each group, *P < .05). Representative results of 3 individual experiments. Data are mean ± SD, *P < .05.
Up-regulating CD55 expression reduces MSC cytotoxicity after their contact with sera
Up-regulating cell-surface complement regulators is one of the attractive strategies to protect self-cells from complement-mediated attack. To test whether up-regulating one of the cell-surface complement regulators in MSCs would reduce MSC cytotoxicity after their contact with serum, we prepared a recombinant adenovirus that expresses human CD55 protein. We transfected MSCs either with the human CD55–expressing adenovirus, or with the empty control adenovirus. After 48 hours, we measured expression levels of human CD55 on the transfected MSC cell surface by flow cytometry, and carried out the same BCECF release-based cytotoxicity assays. These assays showed that transfecting MSCs with the empty control adenovirus had little, if any, effect on CD55 expression levels on MSCs, whereas transfecting MSCs with the CD55-expressing adenovirus significantly increased the levels of CD55 protein on the cell surface (Figure 7C). Correlated with these flow cytometry results, in the cytotoxicity assays, the MSCs with up-regulated CD55 levels showed reduced cytotoxicity compared with MSCs transfected with the control adenovirus (Figure 7D).
We next tested the efficacy of up-regulating CD55 in protecting MSCs from complement-mediated injury in vivo. We first transfected MSCs with the mouse CD55 (daf1)–expressing adenovirus (human CD55 does work well in regulating mouse complement) or the control virus, then labeled the transfected MSCs with BCECF, and infused them (1 × 106/mouse) into WT mice. We assessed the MSCs cytotoxicity in vivo by measuring levels of BCECF leaked into the serum as described in Figure 1. These assays showed that the MSCs with mouse CD55 were better protected from serum-mediated injury than the control MSCs in vivo after infusion (Figure 7E).
Discussion
In this report, we demonstrated that MSCs activated complement on contact with serum, and were injured by the complement activation product MAC despite the presence of all 3 intrinsic cell-surface complement inhibitors. CD55 and CD59 appeared to be more important than CD46 in protecting MSCs from complement-mediated attack in these assays. Up-regulating CD55 levels on MSCs or supplementing an anti-C5 IgG was effective in reducing MSCs cytotoxicity after their contact with serum. In addition, auto-MSCs provoked less intense complement activation and suffered less cellular injury than allo-MSCs after their contact with serum.
MSCs are widely believed in the field to be immunoprivileged because they are immunosuppressive, and they express low levels of major histocompatibility complex (MHC) class I but not MHC class II.2 Because of this belief, and the convenience of preparation, timing, and cost effectiveness, most of the clinical trials on MSCs use allo-MSCs.24 However, it is also commonly observed, both in human and animal studies, that most of the MSCs disappeared within a few days after infusion,24-26 indicating that these cells are rapidly recognized and rejected by the hosts through a yet unknown mechanism(s). The latest disappointing results from MSCs clinical trials further support the suspicion that immediately after infusion, MSC viability and/or activities are markedly reduced, leading to failed treatment efficacy.
Complement, as part of the innate immunity, recognizes anything “foreign” and tries to destroy the invaders through many mechanisms including forming MAC to directly attack the target cells.5 Although MSCs are widely believed to be able to escape from the host adaptive immune system-mediated attack because of their potent immunosuppressive activity, we hypothesized that complement in the serum from the innate immunity is able to recognize, and attack MSCs immediately after their encounter. Indeed, we found that primary MSCs activated complement on contact with the sera, and were injured by the complement activation product MAC both in vitro and in vivo. All 3 complement activation pathways, that is, the classic, the alternative, and the lectin pathways, are involved in the MSC-promoted complement activation, as indicated by the results showing that a deficiency of any one of the pathways reduced, but did not abolish, MSC cytotoxicity after their contact with sera. Depletion (reduction) of IgG levels in the serum reduced and did not abolish MSC cytotoxicity, indicating that naturally existing antibodies in the serum are partially responsible for MSCs injury after infusion. It also appears that the alternative pathway plays a major role in the complement-mediated attack of MSCs because deficiency of this pathway reduced MSC cytotoxicity the most. This is not surprising, given the current knowledge that the alternative pathway not only directly initiates complement activation, but also serves as the amplification loop after complement activation is initiated through the other pathway(s).
Whether auto-MSCs can be injured by complement in the serum and whether these MSCs have any advantages over allo-MSCs were unclear. Our data demonstrated that even auto-MSCs activated complement in the serum, suggesting that the in vitro expanded MSCs were not identical to their original form and that their cell surface was altered enough to be recognized by complement in the serum after the in vitro culture. Nevertheless, our results also showed that auto-MSCs provoked less intense complement activation and suffered less cellular injury after contacting serum than allo-MSCs, suggesting that auto-MSCs could have a longer half-life and better preserved function than allo-MSCs after infusion.
We previously reported that MSCs constitutively produce factor H, a complement inhibitor, which controls complement activation in the fluid phase.11 Our flow cytometry analysis in this study also showed that all of the 3 intrinsic cell-surface complement regulators are present on MSCs. However, complement activated by the MSCs overwhelmed all these existing protecting mechanisms, and resulted in cellular injury. By transfecting MSCs with CD55-expressing adenovirus, we demonstrated that up-regulating CD55 levels on MSCs helped to reduce the severity of MSCs injury after their contact with serum both in vitro and in vivo. These proof-of-concept experiments indicate that up-regulating levels of cell surface complement inhibitors on MSCs could be effective in decreasing MSC cytotoxicity after their infusion. In addition to genetically engineering MSCs, there are other approaches that could also be used to up-regulate cell surface complement regulators on MSCs for clinical applications. For example, it was reported27,28 that statins, a group of FDA-approved drugs for cardiovascular diseases, up-regulate CD55 and CD59 expression on human umbilical vein endothelial cells, and therefore protect these cells from complement mediated attack. These data, taken together, suggest that up-regulating expression levels of cell-surface complement regulators on MSCs by genetic engineering, or by treating the cells/patients with appropriate reagents such as statins, would help to preserve MSC function after their infusion. In addition, screening for MSCs with elevated levels of cell surface complement regulators before large-scale expansion should also be beneficial in achieving the goals of preserving MSCs viability and function after infusion.
We found that incomplete MAC (C5b-8) were enough to induce significant cellular damage in MSCs. This result is consistent with previous reports that C5b-8 are able to induce hemolysis and cytotoxicity,29,30 suggesting that targeting C5, rather than C9, could be effective in protecting MSCs from complement-mediated attack. Indeed, supplementing an anti-C5 IgG significantly reduced MSCs cytotoxicity after their incubation with serum. The FDA has approved an anti-C5 mAb for treating paroxysmal nocturnal hemoglobinuria (PNH) patients in which erythrocytes are lysed by MAC, leading to hemoglobinuria and anemia,31 indicating that this FDA-approved drug could be used in clinic to retain MSC viability and function after their infusion. In addition to the anti-C5 mAb that has been approved for clinical applications, our results suggest that other complement inhibitors that are under development, for example, compstatin,32 recombinant factor H,33 and soluble complement receptor 1,34 could also be developed to improve the efficacy of MSCs in treatment.
In summary, contrary to the current dogma, our data indicate that immediately after infusion, MSCs can be recognized and injured by complement of the host innate immune system. These results argue that autologous MSCs could have advantages over allogeneic MSCs after infusion, and that inhibiting complement activation could be a novel approach to improve current MSC-based therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr David Wald (Director of the MSC Core Facility at National Center of Regenerative Medicine, Case Western Reserve University) for help on MSC isolation and characterization; they also thank Dr Nenoo Rawal (Department of Biochemistry, University of Texas Health Center, Tyler, Texas) for providing the MBL-depleted sera.
This work was supported in part by a grant from the National Institutes of Health (R01NS052471; F.L.), and a pilot grant (F.L.) from the National Center of Regenerative Medicine at Case Western Reserve University.
National Institutes of Health
Authorship
Contribution: F.L. conceived the study, analyzed data, and wrote the paper; and Y.L. performed experiments and analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Feng Lin, Institute of Pathology, Case Western Reserve University School of Medicine, 2085 Adelbert Rd, Rm 306, Cleveland, OH 44106; e-mail: feng.lin@case.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal