Abstract
Adult hematopoiesis occurs primarily in the BM space where hematopoietic cells interact with stromal niche cells. Despite this close association, little is known about the specific roles of osteoblastic lineage cells (OBCs) in maintaining hematopoietic stem cells (HSCs), and how conditions affecting bone formation influence HSC function. Here we use a transgenic mouse model with the ColI(2.3) promoter driving a ligand-independent, constitutively active 5HT4 serotonin receptor (Rs1) to address how the massive increase in trabecular bone formation resulting from increased Gs signaling in OBCs impacts HSC function and blood production. Rs1 mice display fibrous dysplasia, BM aplasia, progressive loss of HSC numbers, and impaired megakaryocyte/erythrocyte development with defective recovery after hematopoietic injury. These hematopoietic defects develop without compensatory extramedullary hematopoiesis, and the loss of HSCs occurs despite a paradoxical expansion of stromal niche cells with putative HSC-supportive activity (ie, endothelial, mesenchymal, and osteoblastic cells). However, Rs1-expressing OBCs show decreased expression of key HSC-supportive factors and impaired ability to maintain HSCs. Our findings indicate that long-term activation of Gs signaling in OBCs leads to contextual changes in the BM niche that adversely affect HSC maintenance and blood homeostasis.
Introduction
Hematologic malignancies account for ∼ 9% of all newly diagnosed cancers in the United States.1 Changes in hematopoiesis also occur in many medical conditions and significantly contribute to morbidity and mortality. Although blood disorders arise primarily from defects in hematopoietic cells, contextual signals from stromal cells in the BM microenvironment or “niche” may also influence disease development.
Adult hematopoiesis occurs in the BM where multipotent hematopoietic stem cells (HSCs) generate all lineages of mature blood cells through a hierarchy of developmentally restricted progenitor populations.2 HSCs reside in specialized niches formed by different stromal populations, including endothelial cells (ECs), mesenchymal stem cells (MSCs), and osteoblastic-lineage cells (OBCs), which express key HSC-supportive factors, including Notch ligands, Cxcl12, and angiopoietin-1 (Angpt1).3,4 Although early mouse studies have implicated mature osteoblasts as important regulators of HSC numbers,5-7 more recent work has shown that immature OBCs and perivascular MSCs are in fact the major BM niche constituents ensuring HSC maintenance.8-10 Coculture experiments have also demonstrated the functional importance of interactions with ECs and OBCs in regulating blood production by HSCs.11,12 These findings indicate that ECs, OBCs, and MSCs are 3 essential BM stromal cell populations that regulate HSC activity and blood homeostasis.
Recent work has also illustrated how changes in BM stromal cells contribute to the development of hematologic diseases. Genetic ablation of the retinoblastoma,13 retinoic acid receptor gamma,14 or miRNA processing enzyme Dicer15 genes in BM stromal cells or osteoprogenitor cells all resulted in dysfunctional BM niches promoting myeloproliferative neoplasms or myelodysplastic syndromes. Furthermore, the loss of Gs G-protein-coupled receptor (GPCR) signaling in osteoprogenitor cells was found to impair B-cell development.16 These examples stress the role of BM stromal niche cells in regulating hematopoiesis and indicate that appropriate GPCR signals are crucial for normal blood development.
Surprisingly little is known about how abnormal Gs-GPCR signaling in osteoblastic cells affects hematopoiesis. Activation of the key osteoblast Gs GPCR parathyroid hormone receptor 1 (PTHR1) by parathyroid hormone (PTH) causes changes in osteoblast proliferation, differentiation, and function.17 Clinically, daily injection of recombinant PTH is used to increase bone formation for osteoporosis treatment.18 Recently, PTH injections were also shown to increase both HSC mobilization and engraftment in mouse models of BM transplantation.5,19,20 These studies have led to clinical trials using PTH to enhance HSC-based therapies21 and raised the exciting possibility of improving HSC function by modulating osteoblast factors, particularly via GPCR signals.
The complexity of the Gsα gene locus, embryonic lethality of Gsα overactivity, and genetic imprinting of this locus pose significant challenges for manipulating Gs signaling in vivo.22 Engineered receptors, such as RASSLs (receptors activated solely by synthetic ligands), are powerful tools for studying GPCR signaling.23 RASSLs no longer respond to endogenous hormones but can be activated by synthetic small-molecule ligands. RASSLs are also small genes easily expressed in constructs and transgenes. We previously showed that the RASSL Rs1 (engineered from the 5HT4 serotonin receptor) had strong basal Gs signaling activity, even in the absence of its ligand.24,25 Expression of Rs1 from the Collagen-1α1 2.3-kb promoter fragment in osteoblastic cells of ColI(2.3)+/Rs1+ transgenic mice (abbreviated as Rs1 mice) dramatically increased trabecular bone formation, obliterated the BM canal, and induced BM fibrosis.25 Here we use the Rs1 mice to investigate how such dramatic BM architectural changes resulting from constitutive Gs signaling in osteoblastic cells affects HSC function and blood production.
Methods
Mice
ColI(2.3)+/Rs1+ (Rs1) mice and control littermates generated by crossing ColI(2.3)-tTA with TetO-Rs1 mice were maintained on the FVB/N-CD45.1 background.25 Reporter mice were generated by crossing ColI(2.3)-tTA with TetO-H2B-GFP26 (JAX) mice. Congenic FVB/N-CD45.2 mice (8-12 weeks old) were used as recipients for transplantation experiments. Rs1 mice received regular chow, allowing basal Rs1 receptor activity to induce Gs signaling in osteoblastic cells.24,25 Six- and 12-week-old mice were used for the majority of experiments because Rs1 mice die or required euthanasia (for spinal stenosis, paralysis, or infection) between 9 and 25 weeks of age.24,25 Male and female mouse data were pooled as no significant sex differences in bone phenotypes were identified.25 For cocultures, HSCs were isolated from wild-type C57BL/6-CD45.2 and β-actin–GFP C57BL/6-CD45.2 transgenic mice.27 Congenic C57BL/6-CD45.1 mice (8-12 weeks old) were used as recipients for transplantation experiments. 5-fluorouracil (5-FU, F8423; Sigma-Aldrich) was administered by intraperitoneal injections as indicated. The Institutional Animal Care and Use Committee at the University of California, San Francisco approved all mouse studies.
Bone analyses
Bone densitometry was performed as described.25 Mid-femur CT scans (50 slices) were performed on a vivaCT40 MicroCT (Scanco; 55-kV x-ray energy, 10.5-μm voxels, 1000-ms integration times). Femur tissue volume (TV), mineralized bone volume (BV), and non-bone volume (TV-BV; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) were determined using segmentation values of 0.7/1/250,25 corresponding to 400 mg hydroxyapatite/cm3, which includes mineralized bone and osteoid. Bones for hematoxylin and eosin staining were decalcified for 12-48 hours in 10% EDTA/PBS before paraffin embedding, sectioning, and staining.
Stromal and hematopoietic cell isolation
Hematopoietic and endosteal BM cells were purified from 12 bones (femurs, tibias, fibulas, humeri, ulnas, radii, and pelvis). Rs1 bones were precrushed by 3 blows of a 0.45-kg hammer and then treated similarly to control bones. After crushing with mortar and pestle, bone chips were washed once in HBSS/2% heat-inactivated FBS to release the loosely adherent BM cells, and several times in HBSS without FBS until the chips were white. These washes were pooled and treated with ACK (150mM NH4Cl, 10mM KHCO3) to lyse red blood cells (RBCs). Residual bone material and dead cells were removed by Ficoll gradient (Histopaque-1119; Sigma-Aldrich). This hematopoietic BM fraction was not collagenase treated and was used in all subsequent hematopoietic analyses. Endosteal stromal cells were released from the hematopoietic-depleted bone chips by digestion with 3 mg/mL type I collagenase (Worthington) and 15 μg/mL DNAse dissolved in HBSS for 1 hour at 37°C at 110 rpm, followed by Ficoll gradient. This stromal fraction was used in all subsequent analyses of endosteal populations. Because CD45+ hematopoietic cells still remain in this fraction, we calculated and applied a corrective efficiency ratio for BM cell isolation (supplemental Figure 2). Splenocytes were obtained by mechanical dissociation followed by ACK treatment. RBC lysis was not performed when analyzing erythrocyte precursors. Tail vein peripheral blood was collected into K2EDTA-coated collection tubes (BD Biosciences) for complete blood counts or into ACK/10mM EDTA buffer for flow cytometry. ELISAs were performed according to manufacturers' instructions (mouse Tpo and Epo, R&D Biosystems; mouse iPTH, Immutopics International). Cellularities were measured using a Vi-Cell automated cell counter (Beckman-Coulter), and complete blood counts were performed on a Hemavet950 analyzer (Drew Scientific).
Flow cytometry
Cell sorting and analysis for hematopoietic28-30 and stromal31,32 BM populations were performed as described in supplemental Table 1. Stained cells resuspended in HBSS/2% FBS/1 μg/mL propidium iodide were isolated or analyzed on a FACSAriaII or LSRII (BD Biosciences), respectively. HSCs were double-sorted for maximum purity. Stromal populations were single-sorted and directly deposited into tissue culture wells. Cells for RNA analysis were collected in Trizol (Invitrogen). For HSC cell cycle status, BM cells were first stained for cell surface markers and then for intracellular Ki67 and DAPI using the Cytofix/Cytoperm Kit (BD Biosciences).
Quantitative RT-PCR
RNA isolated from 600-23 000 purified cells using the Arcterus PicoPure RNA kit was amplified using the Nugen Pico WTA kit and cleaned using the Qiagen QiaQuick PCR purification kit following the manufacturer's protocols. Quantitative RT-PCR on 10 ng of amplified cDNA was performed on an ABI 7900HT thermocycler using TaqMan probesets (Applied BioSystems; supplemental Table 2). Relative expression was calculated using the ΔΔCt method normalized to GAPDH levels for each individual sample measured in triplicate.
Transplantations
Congenic recipients were lethally irradiated at 900 cGy for FVB/N mice and 1100 cGy for C57BL/6 mice delivered in split doses 3 hours apart. Cells were injected into the retro-orbital plexus of the recipients immediately after irradiation, and mice were treated with antibiotics for 6 weeks after transplantation.
Cell culture
Colony-forming unit (CFU) activity was determined in IMDM-based methylcellulose medium (M3231; Stem Cell Technologies) supplemented with SCF, Flt3-L, IL-11, Tpo (25 ng/mL each), IL-3 (10 ng/mL), Epo (4 U/mL), and GM-CSF (20 ng/mL; PeproTech). Colonies were counted after 7 days of culture. Coculture experiments were performed in a 1:1 mix of DMEM and α-modified Eagle medium containing 10% FBS, 1× penicillin/streptomycin, and 50mM 2-mercaptoethanol (coculture medium). Sorted OBCs (2000 cells per well) were cultured for 1 day in a 96-well plate. Nonadherent cells were washed once, and naive HSCs were sorted directly onto the adherent OBCs and grown in coculture medium supplemented with SCF, Flt3-L, and IL11 (25 ng/mL each). After 4 days, all cells (including OBCs) were harvested by vigorous pipetting, stained for CD45, counted, and assessed in methylcellulose for CFU activity. For competitive transplantation experiments, equal numbers of wells from Rs1 and control OBC cocultures were mixed so each recipient received a 1:1 cell ratio. For CFU-fibroblasts (CFU-F), CFU-alkaline phosphatase (CFU-Alk), and CFU-osteoblasts (CFU-OB) assays, 300 OBCs or MSCs were sorted directly into a 6-well plate containing α-modified Eagle medium, 10% FBS, 500 IU penicillin, 500 μg/ml streptomycin, and 50mM 2-mercaptoethanol (CFU-medium). From day 2 onward, CFU-Alk and CFU-OB cultures were induced by adding 3mM β-glycerophosphate (G9891; Sigma-Aldrich) and 10 mg/mL L-ascorbic acid 2-phosphate (A8960; Sigma-Aldrich) to the CFU-medium. Medium was replaced every 2-3 days. At day 11, CFU-F and CFU-Alk cultures were stained with Giemsa-Wright and alkaline phosphatase, respectively. At day 21, CFU-OB cultures were stained by von Kossa.
Statistical analysis
P values were calculated using the Student t test, and differences with P ≤ .05 were considered statistically significant.
Results
BM aplasia in mice with increased osteoblastic Gs signaling
Rs1 mice progressively develop a dramatic bone phenotype characterized by increased trabecular bone formation, dense fibrocellular infiltrates, and decreased hematopoietic cells within the putative BM space (Figure 1A-F).25 Previous analysis by von Kossa/tetrachrome staining and synchrotron radiation imaging revealed under-mineralization and disorganized structure in Rs1 bones.25,33 Micro-CT analyses at femoral mid-diaphyses detecting both bone and osteoid showed increased TV, mineralized BV, and BV/TV fraction in 15-week-old Rs1 mice, but no decrease in non-BV (TV-BV; Figure 1G; supplemental Figure 1). These results suggest that the total potential space for cellular BM components at the mid-diaphysis is not reduced in Rs1 mice despite massive changes in overall bone architecture. However, the absolute numbers of hematopoietic BM cells released by extensive washing from the crushed bones of 6-week-old and 12-week-old Rs1 mice were significantly reduced compared with control bones (Figure 1H). Although BM cell isolation was less efficient in Rs1 bones, 12-week-old Rs1 mice still displayed 73% fewer hematopoietic BM cells than control mice when corrected for this difference in extraction efficiency (supplemental Figure 2). Flow cytometric analysis of cells released from collagenase-treated bone chips revealed a 4-fold increase in the number of endosteal BM Lin−/CD45− stromal cells in 12-week-old Rs1 mice (Figure 1I). This finding indicates that stromal components infiltrate into the nonbone space in Rs1 mice. Thus, although Rs1 mice may have more potential space for BM components, the expanded stromal components and the increased nonmineralized matrix deposited by these cells reduce the capacity for hematopoietic cells, inducing BM aplasia.
Changes in the BM cavity of Rs1 mice. (A) Representative photographs of femurs from 6-week-old (6 wk) and 12-week-old (12 wk) control (Ctrl) and ColI(2.3)+/Rs1+ (Rs1) mice. Scale bar represents 1 cm. (B) Dual-energy x-ray absorptiometry measurements of bone mineral density (BMD) in age-matched Ctrl and Rs1 littermates (n = 6-15 mice per group). (C-F) Hematoxylin and eosin staining of decalcified femurs from 12-weeks-old Ctrl and Rs1 mice. Low magnification (C) showing loss of the normal BM canal and gross morphologic bone changes. Scale bar represents 2 mm. High magnification showing hematopoietic BM cells and cortical bone in Ctrl (D) and Rs1 bone containing fibrous infiltrate and disorganized trabeculae (E) and hematopoietic BM cells (F) intermingled with fibrocellular infiltrate. c indicates cortical bone; bm, BM space; t, trabecular bone; and f, fibrous stromal cells. Scale bar represents 100 μm. (G) Micro-CT analysis on femurs of 15-week-old (15 wk) Ctrl (n = 4) and Rs1 (n = 5) mice. Scale bar represents 1 mm. TV and mineralized BV were determined on 50 mid-femur slices. (H) Enumeration of BM hematopoietic cell numbers released by crushing and extensive washing of bones isolated from 6-week-old and 12-week-old Ctrl and Rs1 mice (n = 6-13 per group). (I) Enumeration of BM Lin−/CD45− endosteal stromal cells released by collagenase-digestion from the crushed bones of 12-week-old control and Rs1 mice. Data are mean ± SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001.
Changes in the BM cavity of Rs1 mice. (A) Representative photographs of femurs from 6-week-old (6 wk) and 12-week-old (12 wk) control (Ctrl) and ColI(2.3)+/Rs1+ (Rs1) mice. Scale bar represents 1 cm. (B) Dual-energy x-ray absorptiometry measurements of bone mineral density (BMD) in age-matched Ctrl and Rs1 littermates (n = 6-15 mice per group). (C-F) Hematoxylin and eosin staining of decalcified femurs from 12-weeks-old Ctrl and Rs1 mice. Low magnification (C) showing loss of the normal BM canal and gross morphologic bone changes. Scale bar represents 2 mm. High magnification showing hematopoietic BM cells and cortical bone in Ctrl (D) and Rs1 bone containing fibrous infiltrate and disorganized trabeculae (E) and hematopoietic BM cells (F) intermingled with fibrocellular infiltrate. c indicates cortical bone; bm, BM space; t, trabecular bone; and f, fibrous stromal cells. Scale bar represents 100 μm. (G) Micro-CT analysis on femurs of 15-week-old (15 wk) Ctrl (n = 4) and Rs1 (n = 5) mice. Scale bar represents 1 mm. TV and mineralized BV were determined on 50 mid-femur slices. (H) Enumeration of BM hematopoietic cell numbers released by crushing and extensive washing of bones isolated from 6-week-old and 12-week-old Ctrl and Rs1 mice (n = 6-13 per group). (I) Enumeration of BM Lin−/CD45− endosteal stromal cells released by collagenase-digestion from the crushed bones of 12-week-old control and Rs1 mice. Data are mean ± SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001.
Impaired BM megakaryopoiesis and erythropoiesis
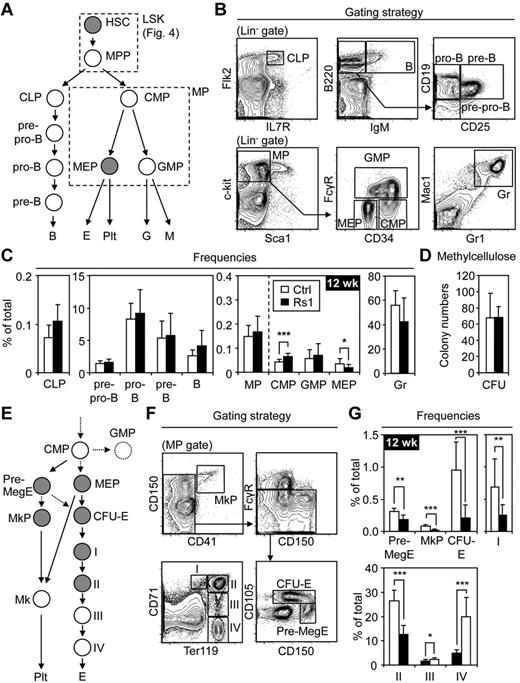
We performed immunophenotyping to determine whether specific blood lineages were affected in Rs1 mice (Figure 2A-B). Although a mild decrease in mature B-cell frequencies was observed in 6-week-old Rs1 BM (supplemental Figure 3), this resolved in 12-week-old mice (Figure 2C). Other lymphoid progenitor populations, including CLP, pre-pro B, pro-B, pre-B, and immature B, were unchanged (Figure 2C). The frequency of total myeloid progenitors (MP) was also unchanged, with methylcellulose cultures of unfractionated control or Rs1 BM cells showing no differences in CFU activity (Figure 2C-D; supplemental Figure 3). Additional fractionation of the MP compartment revealed increases in CMP frequency but normal GMP and Gr frequencies, indicating unaffected myelopoiesis (Figure 2C; supplemental Figure 3). Surprisingly, Rs1 mice showed decreased MEP frequencies suggesting a developmental defect in the megakaryocyte and erythrocyte lineages. Additional fractionation strategies (Figure 2E-F) confirmed a striking decrease in MkP, Pre-MegE, and CFU-E frequencies (Figure 2G). The loss of erythrocyte precursors was also reflected by the decreased frequencies of pro-erythroblasts (I) and basophilic erythroblasts (II). In contrast, the frequencies of late basophilic/polychromatophilic erythroblasts (III) and orthochromatic erythroblasts (IV) were increased, possibly as attempted compensation for the decrease in erythrocyte precursors. However, in absolute numbers, all stages of BM erythropoiesis and megakaryopoiesis were reduced (data not shown). Thus, constitutive Gs activation in OBCs of Rs1 mice does not affect lymphopoiesis or myelopoiesis but specifically decreases megakaryocyte and erythrocyte progenitor numbers.
Lineage-specific hematopoietic defects in the BM of Rs1 mice. Twelve-week-old (12 wk) control (Ctrl) and ColI(2.3)+/Rs1+ (Rs1) mice (n = 8-13 per group) were used for these analyses. (A) Schematic overview and (B) gating strategy for the different hematopoietic lineages investigated (gray represents populations decreased in frequency in Rs1 mice). CLP indicates common lymphoid progenitor; B, B-cell; CMP, common myeloid progenitor; GMP, granulocyte/macrophage progenitor; MEP, megakaryocyte/erythrocyte progenitor; E, erythrocyte; Plt, platelet; Gr, granulocyte; and M, macrophage. The myeloid progenitor (MP) population containing CMPs, GMPs, and MEPs and the LSK population containing HSCs and MPPs are boxed. (C) Frequencies of the indicated populations in the BM. (D) Methylcellulose read-out for myeloid CFU activity in 12 500 unfractionated Ctrl and Rs1 BM cells. (E) Schematic overview and (F) gating strategy for detailed analyses of megakaryopoiesis and erythropoiesis (gray represents populations decreased in frequency in Rs1 mice). Pre-MegE indicates megakaryocyte/erythrocyte precursor; MkP, megakaryocyte precursor; Mk, megakaryocyte; CFU-E, colony-forming unit-erythrocyte; I, pro-erythroblast; II, basophilic erythroblast; III, late basophilic/polychromatophilic erythroblast; and IV, orthochromatic erythroblast. (G) Frequencies of the indicated populations in the BM. Data are mean ± SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001.
Lineage-specific hematopoietic defects in the BM of Rs1 mice. Twelve-week-old (12 wk) control (Ctrl) and ColI(2.3)+/Rs1+ (Rs1) mice (n = 8-13 per group) were used for these analyses. (A) Schematic overview and (B) gating strategy for the different hematopoietic lineages investigated (gray represents populations decreased in frequency in Rs1 mice). CLP indicates common lymphoid progenitor; B, B-cell; CMP, common myeloid progenitor; GMP, granulocyte/macrophage progenitor; MEP, megakaryocyte/erythrocyte progenitor; E, erythrocyte; Plt, platelet; Gr, granulocyte; and M, macrophage. The myeloid progenitor (MP) population containing CMPs, GMPs, and MEPs and the LSK population containing HSCs and MPPs are boxed. (C) Frequencies of the indicated populations in the BM. (D) Methylcellulose read-out for myeloid CFU activity in 12 500 unfractionated Ctrl and Rs1 BM cells. (E) Schematic overview and (F) gating strategy for detailed analyses of megakaryopoiesis and erythropoiesis (gray represents populations decreased in frequency in Rs1 mice). Pre-MegE indicates megakaryocyte/erythrocyte precursor; MkP, megakaryocyte precursor; Mk, megakaryocyte; CFU-E, colony-forming unit-erythrocyte; I, pro-erythroblast; II, basophilic erythroblast; III, late basophilic/polychromatophilic erythroblast; and IV, orthochromatic erythroblast. (G) Frequencies of the indicated populations in the BM. Data are mean ± SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001.
No compensatory extramedullary hematopoiesis
We then asked whether Rs1 mice developed extramedullary hematopoiesis to compensate for the BM aplasia and loss of megakaryocyte/erythrocyte progenitors. Both control and Rs1 spleens appeared similar in size and cellularity (supplemental Figure 4A-B), and neither kidneys nor livers of Rs1 mice showed histologic evidence of extramedullary hematopoiesis (data not shown). Although 6-week-old Rs1 spleens had mildly decreased T-cell numbers, this resolved in 12-week-old Rs1 mice, and no other populations of progenitors or mature myeloid and lymphoid cells were affected (supplemental Figure 4C-D; and data not shown). Transplantation of 1 × 107 spleen cells from 12-week-old Rs1 or control mice into lethally irradiated hosts yielded similar levels of chimerism (data not shown), confirming the absence of splenic hematopoiesis in Rs1 mice. Frequencies of MEPs and immature erythrocyte progenitor populations were also unchanged in Rs1 spleens (supplemental Figure 4E-F; and data not shown). As a consequence of normal splenic erythropoiesis, 12-week-old Rs1 mice showed normal RBC counts and no sign of peripheral anemia or thrombocytopenia (supplemental Figure 4G). Thus, despite severe developmental defects in megakaryocyte and erythrocyte lineages in the BM, no compensatory extramedullary hematopoiesis or altered levels of circulating blood cells was observed at steady state in Rs1 mice.
Defective RBC and platelet recovery after acute hematopoietic injury
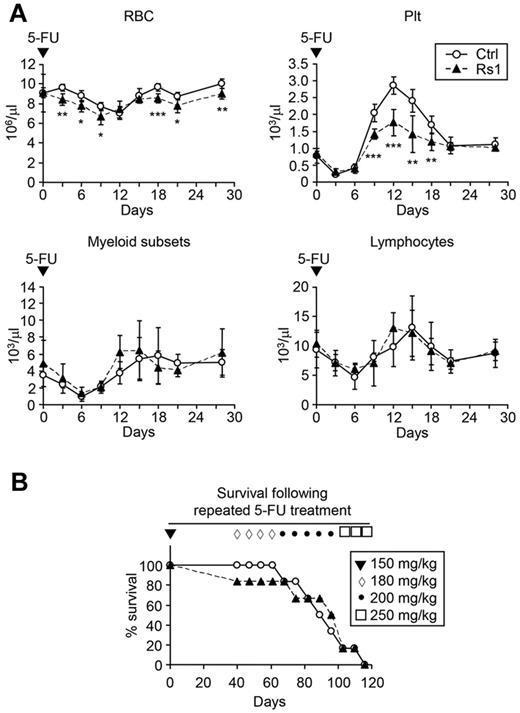
To determine whether the BM defects compromised the ability of Rs1 mice to respond to hematopoietic injury, we challenged 9-week-old mice with a single dose of 5-FU, a myelosuppressive agent that kills cycling hematopoietic cells (Figure 3A). Although similar decline and recovery were observed in lymphoid and myeloid cells, Rs1 mice showed more rapid declines and incomplete recovery of RBC counts compared with control mice. Platelet recovery was also significantly attenuated in Rs1 mice. We then injected mice weekly with escalating doses of 5-FU to model repeated hematopoietic injury but observed no major differences in the survival rates of Rs1 and control mice (Figure 3B). These results illustrate the resilience of the blood system in Rs1 mice and indicate that the BM megakaryocyte/erythrocyte defects contribute to impaired RBC and platelet recovery after acute hematopoietic injury.
Impaired recovery of Rs1 mice after acute hematopoietic injury. Nine-week-old control (Ctrl) and ColI(2.3)+/Rs1+ (Rs1) mice (n = 6 per group) were used to start these experiments. (A) Hematopoietic recovery after a single injection (150 mg/kg) of 5-FU. Blood was sampled and analyzed by automated complete blood count for the indicated populations. Plt indicates platelets; and myeloid subsets, eosinophil/basophil/neutrophil/monocyte. (B) Forty days after the first 5-FU injection, mice were injected every 7 days with escalating doses of 5-FU (ranging from 180 mg/kg to 250 mg/kg, as indicated) and followed for survival. Data are mean ± SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001.
Impaired recovery of Rs1 mice after acute hematopoietic injury. Nine-week-old control (Ctrl) and ColI(2.3)+/Rs1+ (Rs1) mice (n = 6 per group) were used to start these experiments. (A) Hematopoietic recovery after a single injection (150 mg/kg) of 5-FU. Blood was sampled and analyzed by automated complete blood count for the indicated populations. Plt indicates platelets; and myeloid subsets, eosinophil/basophil/neutrophil/monocyte. (B) Forty days after the first 5-FU injection, mice were injected every 7 days with escalating doses of 5-FU (ranging from 180 mg/kg to 250 mg/kg, as indicated) and followed for survival. Data are mean ± SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001.
Specific decrease in phenotypic and functional HSCs
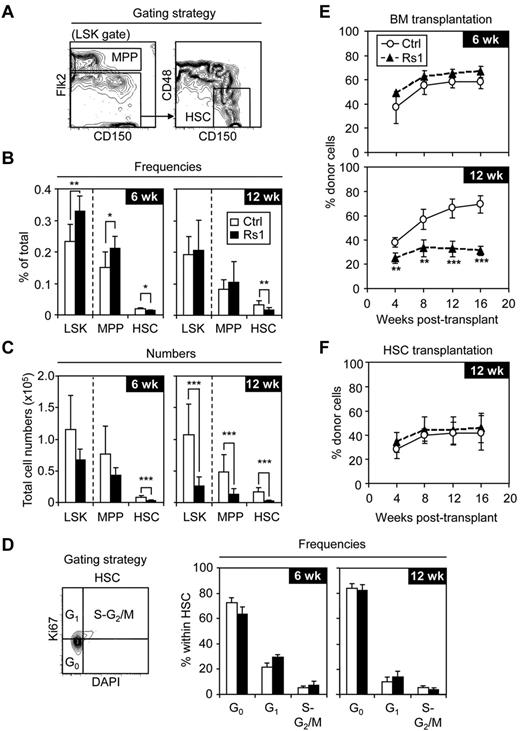
We investigated whether the immature stem and progenitor BM compartments were affected in Rs1 mice. Detailed phenotypic analyses of Lin−/Sca-1+/c-Kit+ (LSK) cells using Flk2, CD150, and CD48 markers (Figure 4A) revealed a progressive loss of Flk2−/CD150+/CD48− LSK HSCs in both frequency (Figure 4B) and absolute numbers (Figure 4C) in 6-week-old and 12-week-old Rs1 mice. This striking loss of HSCs was not the result of their inefficient isolation from crushed Rs1 bones (supplemental Figure 5A-B). In contrast, 6-week-old Rs1 mice showed significantly increased frequencies of LSK cells and Flk2+ LSK multipotent progenitors (MPPs), ensuring maintenance of relatively normal total numbers of these 2 subsets. By 12 weeks, the frequencies of LSKs and MPPs returned to normal (Figure 4B), whereas the absolute numbers of these populations became severely reduced in Rs1 mice (Figure 4C). Cell cycle analyses (Figure 4D) showed a trend toward decreased G0 and increased G1-S-G2/M frequencies in HSCs isolated from 6-week-old Rs1 mice, which was not apparent in HSCs isolated from 12-week-old Rs1 mice. These results demonstrate that Rs1 mice progressively lose their HSC population in the BM, and suggest that HSCs may initially attempt to compensate for the loss of BM cells by increasing their proliferation and differentiation toward MPPs. However, this compensatory mechanism is not occurring at later times when both HSCs and MPPs are actually decreased, leading to significantly lower numbers of all immature stem and progenitor cells in 12-week-old Rs1 mice.
Loss of HSC numbers in Rs1 mice. Six-week-old (6 wk) and 12-week-old (12 wk) control (Ctrl) and ColI(2.3)+/Rs1+ (Rs1) mice (n = 6-13 per group) were used for these analyses. (A) Gating strategy, (B) frequencies, and (C) total cell numbers for the indicated BM stem and multipotent progenitor cells. (D) Cell cycle analyses. Gating strategy (left) and frequencies (right) of HSCs in G0, G1, and S-G2/M phases of the cell cycle. Lethally irradiated FVB/N-CD45.2 congenic recipients (n = 4 or 5 per group) were transplanted with either (E) 1 × 106 CD45.1 BM cells or (F) 150 CD45.1 purified HSCs together with 300 000 Sca-1–depleted CD45.2 helper BM cells. Transplanted mice were bled every 4 weeks and analyzed for the percentage of donor (CD45.1+) chimerism in the peripheral blood. Data are mean ± SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001.
Loss of HSC numbers in Rs1 mice. Six-week-old (6 wk) and 12-week-old (12 wk) control (Ctrl) and ColI(2.3)+/Rs1+ (Rs1) mice (n = 6-13 per group) were used for these analyses. (A) Gating strategy, (B) frequencies, and (C) total cell numbers for the indicated BM stem and multipotent progenitor cells. (D) Cell cycle analyses. Gating strategy (left) and frequencies (right) of HSCs in G0, G1, and S-G2/M phases of the cell cycle. Lethally irradiated FVB/N-CD45.2 congenic recipients (n = 4 or 5 per group) were transplanted with either (E) 1 × 106 CD45.1 BM cells or (F) 150 CD45.1 purified HSCs together with 300 000 Sca-1–depleted CD45.2 helper BM cells. Transplanted mice were bled every 4 weeks and analyzed for the percentage of donor (CD45.1+) chimerism in the peripheral blood. Data are mean ± SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001.
We next assessed whether this loss of phenotypic HSCs in Rs1 mice reflected a functional decrease by transplanting 1 million unfractionated BM cells into lethally irradiated hosts (Figure 4E). Whereas recipients of 6-week-old control or Rs1 BM cells showed similar levels of chimerism, recipients of 12-week-old Rs1 BM cells displayed significantly reduced reconstitution compared with mice transplanted with control BM cells. To directly assess whether HSCs from Rs1 mice are functionally impaired, we transplanted 125 HSCs isolated from 12-week-old control or Rs1 mice into lethally irradiated hosts (Figure 4F). In both cases, we observed similar levels of chimerism and multilineage reconstitution. Furthermore, similar levels of chimerism were observed in competitive transplantation where 25 Rs1 or control HSCs were directly competed against 25 wild-type HSCs (supplemental Figure 5C). These results confirm that 12-week-old Rs1 mice have reduced HSC numbers and demonstrate that the remaining HSCs are not functionally altered on a per-cell basis.
Increased numbers of stromal BM niche cells
To determine how BM stromal cells with HSC-supportive capacity were affected in Rs1 mice, we used established flow cytometry protocols identifying Lin−/CD45−/CD31−/CD51+/Sca-1− OBCs, Lin−/CD45−/CD31−/CD51+/Sca-1+ MSCs, and Lin−/CD45−/CD31+/Sca-1+ ECs within the endosteal stromal compartment28,31 (Figure 5A). We first confirmed that only ColI(2.3)-expressing OBCs expressed Rs1 using ColI(2.3)-tTA/TetO-H2B-GFP double-transgenic reporter mice (Figure 5A-B). We then profiled these endosteal stromal populations in 12-week-old mice and found a dramatic increase in the number of OBCs, ECs, and MSCs in Rs1 bones (Figure 5C). These results indicate that the HSC loss observed in Rs1 mice is accompanied by a paradoxical expansion of stromal BM niche cells with putative HSC supportive activity, including Rs1-expressing OBCs.
Expansion of BM stromal cells in Rs1 mice. Nine-week-old (9 wk) and 12-week-old (12 wk) control (Ctrl) and ColI(2.3)+/Rs1+ (Rs1) mice (n = 4-12 per group) were used for these analyses. (A) Gating strategy used to identify subpopulations of endosteal BM stromal cells (top row) and to monitor ColI(2.3) promoter activity in ColI(2.3)-tTA/TetO-H2B-GFP double-transgenic reporter mice (bottom row). (B) Frequencies of stromal BM cell subpopulations expressing GFP in ColI(2.3)-tTA/TetO-H2B-GFP mice (n = 4). (C) Total cells for the indicated endosteal BM stromal cells. (D) Quantitative RT-PCR analysis of purified OBCs for expression of the indicated osteoblastic lineage genes. Data are expressed as log2 fold relative to the average of the Ctrl samples (set to 0). Averages are shown as black bars. (E-F) Frequencies of CFU-F, CFU-Alk, and CFU-OB formed by OBCs (E) and MSCs (F) isolated from 12-week-old Ctrl and Rs1 mice. Data are mean ± SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001.
Expansion of BM stromal cells in Rs1 mice. Nine-week-old (9 wk) and 12-week-old (12 wk) control (Ctrl) and ColI(2.3)+/Rs1+ (Rs1) mice (n = 4-12 per group) were used for these analyses. (A) Gating strategy used to identify subpopulations of endosteal BM stromal cells (top row) and to monitor ColI(2.3) promoter activity in ColI(2.3)-tTA/TetO-H2B-GFP double-transgenic reporter mice (bottom row). (B) Frequencies of stromal BM cell subpopulations expressing GFP in ColI(2.3)-tTA/TetO-H2B-GFP mice (n = 4). (C) Total cells for the indicated endosteal BM stromal cells. (D) Quantitative RT-PCR analysis of purified OBCs for expression of the indicated osteoblastic lineage genes. Data are expressed as log2 fold relative to the average of the Ctrl samples (set to 0). Averages are shown as black bars. (E-F) Frequencies of CFU-F, CFU-Alk, and CFU-OB formed by OBCs (E) and MSCs (F) isolated from 12-week-old Ctrl and Rs1 mice. Data are mean ± SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001.
We then performed quantitative RT-PCR analyses on OBCs isolated from 12-week-old control and Rs1 mice (Figure 5D). Whereas Rs1 OBCs showed higher expression of the MSC marker Nestin, the osteoblast/chrondrocyte transcription factor Runx2, and the early osteoblast progenitor transcription factor Osterix (Osx), no changes were observed in the expression of mature osteoblast markers, including Collagen 1α1 (ColIα1), Osteopontin (Opn), and Osteocalcin (OC). These molecular data confirm previous observations that expression of Rs1 in osteoblastic cells enriches for OBCs with an immature phenotype.25,33 However, no difference in maturity status was detected when control and Rs1 OBCs were compared in in vitro functional assays for CFU-F, CFU-Alk, and CFU-OB (Figure 5E). In addition, no difference in differentiation potential was observed between control and Rs1 MSCs (Figure 5F). These results indicate that Rs1-mediated Gs signaling considerably increases the numbers of OBCs without affecting their overall differentiation properties.
Decreased HSC-supportive activity
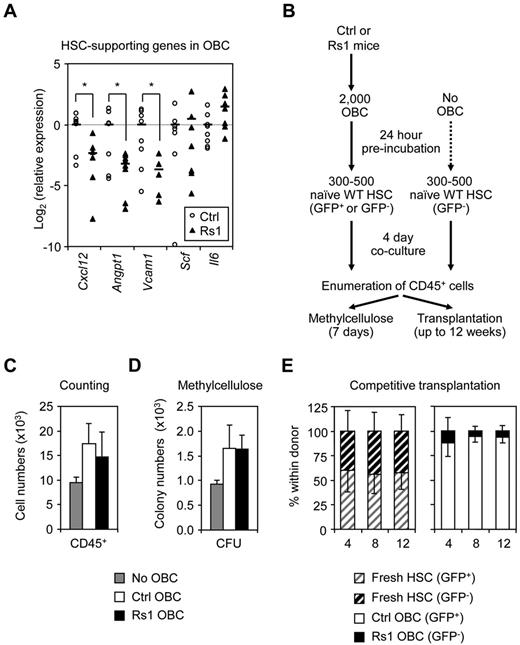
Because of the early morbidity/mortality of Rs1 mice, we were unable to directly assess their BM niche properties by transplanting wild-type HSCs into Rs1 hosts. Instead, we investigated the HSC-supportive ability of purified Rs1-expressing OBCs. Quantitative RT-PCR analyses of OBCs isolated from 12-week-old mice revealed a striking decrease in the expression of HSC maintenance genes, including Cxcl12, Angpt1, and Vcam1, in Rs1 OBCs (Figure 6A). In contrast, expression of the pan-hematopoietic cytokine Scf and myeloid differentiation cytokine Il-6 were unchanged, consistent with the overall normal myeloid differentiation observed in Rs1 mice. We then performed short-term coculture experiments where naive WT HSCs were grown for 4 days with or without OBCs isolated from 12-week-old control or Rs1 mice, then assessed for hematopoietic production, CFU activity, and HSC function in transplantation experiments (Figure 6B). As expected, HSCs cocultured with OBCs showed more hematopoietic differentiation and higher CFU potential than HSCs cultured without OBCs, and coculture with Rs1 OBCs did not change these differentiation properties (Figure 6C-D). We then injected equal numbers of progeny from CD45.2+/GFP− HSCs cocultured with Rs1 OBCs together with progeny from CD45.2+/GFP+ HSCs cocultured with control OBCs into lethally irradiated CD45.1+ congenic hosts (Figure 6E). In this competitive setting, HSCs cultured with Rs1 OBCs were severely outcompeted by HSCs cultured with control OBCs, as evidenced by the increased contribution of GFP+ cells to donor chimerism. We also directly injected the progeny of HSCs cocultured with Rs1 or control OBCs into individual recipient mice and confirmed a trend toward decreased engraftment from HSC-derived cells cocultured with Rs1 OBCs (supplemental Figure 5D). Thus, despite having similar abilities to promote HSC differentiation, Rs1 OBCs are impaired in their ability to maintain HSCs probably because of their decreased expression of HSC-supportive factors.
Defective HSC-supportive function by Rs1 OBCs. Twelve-week-old control (Ctrl) and ColI(2.3)+/Rs1+ (Rs1) mice (n = 5-12 per group) were used for these analyses. (A) Quantitative RT-PCR analysis of purified OBCs for expression of the indicated HSC-supporting genes. Data are expressed as log2 fold relative to the average of the Ctrl samples (set to 0). Averages are shown as black bars. (B) Schematic of the coculture experiment of wild-type (WT) and β-actin-GFP C57BL/6-CD45.2 HSCs (500: counting and methylcellulose; 300: transplantation) with or without Ctrl or Rs1 OBCs. (C) Enumeration of CD45+ hematopoietic cells per well. (D) Methylcellulose read-out for myeloid CFU activity per well. (E) Competitive transplantations. A 1:1 ratio of freshly isolated HSCs (150 GFP+:150 GFP−) or mixed wells containing the progeny of HSCs cocultured on Ctrl (GFP+) or Rs1 (GFP−) OBCs (300 HSC-derived cell equivalent) were transplanted into lethally irradiated C57BL/6-CD45.1 recipient mice together with 300 000 Sca-1–depleted CD45.1 helper BM cells (n = 3-5 per group). Transplanted mice were bled every 4 weeks and analyzed for the percentage of GFP contribution to donor (CD45.2+) chimerism in the peripheral blood. Data are mean ± SD. *P ≤ .05.
Defective HSC-supportive function by Rs1 OBCs. Twelve-week-old control (Ctrl) and ColI(2.3)+/Rs1+ (Rs1) mice (n = 5-12 per group) were used for these analyses. (A) Quantitative RT-PCR analysis of purified OBCs for expression of the indicated HSC-supporting genes. Data are expressed as log2 fold relative to the average of the Ctrl samples (set to 0). Averages are shown as black bars. (B) Schematic of the coculture experiment of wild-type (WT) and β-actin-GFP C57BL/6-CD45.2 HSCs (500: counting and methylcellulose; 300: transplantation) with or without Ctrl or Rs1 OBCs. (C) Enumeration of CD45+ hematopoietic cells per well. (D) Methylcellulose read-out for myeloid CFU activity per well. (E) Competitive transplantations. A 1:1 ratio of freshly isolated HSCs (150 GFP+:150 GFP−) or mixed wells containing the progeny of HSCs cocultured on Ctrl (GFP+) or Rs1 (GFP−) OBCs (300 HSC-derived cell equivalent) were transplanted into lethally irradiated C57BL/6-CD45.1 recipient mice together with 300 000 Sca-1–depleted CD45.1 helper BM cells (n = 3-5 per group). Transplanted mice were bled every 4 weeks and analyzed for the percentage of GFP contribution to donor (CD45.2+) chimerism in the peripheral blood. Data are mean ± SD. *P ≤ .05.
Discussion
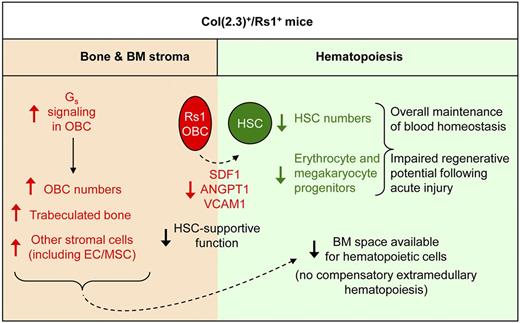
Elucidating the complex interplay between BM hematopoietic and stromal compartments is crucial for understanding normal blood development and identifying new treatments for hematologic diseases. Because OBCs are essential components of the BM HSC niche, we initially hypothesized that increased Gs signaling in OBCs, and the associated increase in OBCs and bone mass, may improve the hematopoietic environment by promoting HSC maintenance and function. Instead, we found that Rs1-induced Gs signaling in OBCs had unexpected adverse consequences for blood production, with lineage-specific defects in erythrocyte and platelet production that were aggravated upon hematopoietic injury. We also uncovered that continuous Gs signaling resulted in an accumulation of OBCs with decreased expression of key HSC-supportive factors leading to compromised HSC maintenance in the BM (Figure 7).
Effect of constitutive Rs1-mediated Gs signaling in OBCs on hematopoiesis. Model summarizing the extensive changes in the bone and BM stromal of ColI(2.3)+/Rs1+ (Rs1) mice and their consequences for HSC maintenance and blood development. OBC-specific expression of Rs1 increases Gs signaling and expands the number of immature osteoblasts leading to increased trabecular bone formation. This also results indirectly in increased numbers of MSCs and ECs. Together, these expanded BM stromal cells restrict the space available for hematopoietic cells and lead to BM aplasia, which is not compensated by extramedullary hematopoiesis. Increased Gs signaling in Rs1-expressing OBCs decreases their expression of key HSC-maintenance genes, including Cxcl12, Angpt1, and Vcam1, and impairs their HSC-supportive function leading to a severe loss of HSC numbers. Rs1 mice also show diminished production of megakaryocyte and erythrocyte progenitors through a mechanism that still largely remains to be elucidated but involves decreased levels of circulating Tpo. As a consequence, Rs1 mice have impaired regenerative potential after acute injury but overall preserved blood function probably because of the contribution of splenic hematopoiesis.
Effect of constitutive Rs1-mediated Gs signaling in OBCs on hematopoiesis. Model summarizing the extensive changes in the bone and BM stromal of ColI(2.3)+/Rs1+ (Rs1) mice and their consequences for HSC maintenance and blood development. OBC-specific expression of Rs1 increases Gs signaling and expands the number of immature osteoblasts leading to increased trabecular bone formation. This also results indirectly in increased numbers of MSCs and ECs. Together, these expanded BM stromal cells restrict the space available for hematopoietic cells and lead to BM aplasia, which is not compensated by extramedullary hematopoiesis. Increased Gs signaling in Rs1-expressing OBCs decreases their expression of key HSC-maintenance genes, including Cxcl12, Angpt1, and Vcam1, and impairs their HSC-supportive function leading to a severe loss of HSC numbers. Rs1 mice also show diminished production of megakaryocyte and erythrocyte progenitors through a mechanism that still largely remains to be elucidated but involves decreased levels of circulating Tpo. As a consequence, Rs1 mice have impaired regenerative potential after acute injury but overall preserved blood function probably because of the contribution of splenic hematopoiesis.
At first, our findings appeared to contradict a prior study where a constitutively active PTHR1 receptor (caPTHR1) expressed from the same ColI(2.3) promoter expanded both osteoblast and HSC numbers.5 However, significant differences in methodologies and bone phenotype severity probably account for this discrepancy. First, the HSC expansion reported in ColI(2.3)-caPTHR1 mice was defined as increased LSK BM cells with increased reconstitution at 8 weeks after transplantation, measured by RT-PCR of Y-chromosome donor chimerism. Although these approaches were state-of-the art techniques at that time, they unfortunately did not exclude non–self-renewing short-term HSCs and MPPs (contained in the LSK compartment) as contributors to the increased blood donor chimerism. Indeed, we also observed increased LSK cells in young (6 weeks) Rs1 mice, although we did not find increased chimerism on transplantation using CD45.1/CD45.2 congenic markers. Furthermore, analyses of older Rs1 mice (12 weeks) with more advanced bone phenotypes showed that the actual number of functional long-term HSCs contained in the LSK fraction was decreased. Studies on the ColI(2.3)-caPTHR1 mice confirming increased long-term HSC numbers using newer phenotypic definitions and more current transplantation approaches have not been published yet. It is also possible that the severity of the bone phenotype directly contributes to the observed differences in HSC numbers. Both ColI(2.3)-caPTHR1 and Rs1 mice have increased fibrous cells and trabecular bone formation.5,25 Whereas caPTHR1 signaling appears more similar to pharmacologic PTH treatment (Laura Calvi, written personal communication, March 9, 2012) or hyperparathyroidism,34 Rs1 signaling is stronger and induces a fibrous dysplasia-like disease35 in Rs1 mice far more severe than that observed in ColI(2.3)-caPTHR1 mice. Therefore, a mild increase in OBC numbers and bone trabeculation may result in increased HSC numbers, whereas a more dramatic increase might be detrimental for hematopoiesis. This possibility is supported by a recent study expressing caPTHR1 in osteocytes using the DMP1 promoter,36 which induced more bone formation akin to the Rs1 mice. The DMP1-caPTHR1 mice showed increased numbers of osteoblastic cells, impaired HSC supportive activity, and decreased numbers of functional HSCs,36 consistent with our findings. Together, these different mouse models illustrate that the level of GPCR signaling and the specific osteoblastic populations where GPCR signaling is activated are crucial determinants for the HSC supportive function of the BM microenvironment.
One unexpected finding in our Rs1 mice was the absence of compensatory extramedullary hematopoiesis despite severe BM aplasia. This contrasts sharply with the DMP1-caPTHR1 mice36 and mice with disrupted BM microenvironments that develop myeloid diseases,13-15,37 which readily show extramedullary hematopoiesis. Although the increased trabecular bone in Rs1 mice creates more space for BM components, we did not observe hematopoietic cell expansion. Rather, we found a striking increase in OBC, MSC, and EC numbers despite OBC-restricted Rs1 expression. This probably involves indirect crosstalk between these different stromal populations, suggesting that GPCR signaling in OBCs is important for controlling the number of endosteal ECs and MSCs. It also suggests that changes in the cellular environment, rather than changes in physical space, may be more important in determining whether extramedullary hematopoiesis will occur.
Surprisingly, Rs1 mice showed progressive loss of HSCs despite large increases in stromal BM cells with potential HSC-supportive ability. Although our results suggest that defective support by Rs1-expressing OBCs contributes to this hematopoietic phenotype, we cannot exclude that MSCs and ECs could also have defective supportive capability, thus contributing to the loss of HSCs in Rs1 mice. Here we directly show that OBCs from Rs1 mice are less supportive for HSC maintenance than OBCs from control mice using in vitro coculture assays. This decreased HSC-supportive ability probably results from the compromised expression by Rs1 OBCs of key HSC-supportive factors, including Cxcl12, Angpt1, and Vcam1, and contributes to the HSC depletion occurring over time in Rs1 mice. Loss of quiescence and increased proliferation can also lead to HSC exhaustion38 ; however, the limited and transient changes in HSC cell cycle distribution we observed in Rs1 mice make this an unlikely explanation. Furthermore, we found that the HSCs remaining in the BM niche of Rs1 mice are not functionally altered as measured in transplantation experiments. This may account for the surprising resilience of blood production in Rs1 mice despite severe HSC depletion and BM aplasia. Our results demonstrate that constitutive Gs activation in OBCs renders the BM microenvironment refractory to HSC maintenance without compromising HSC self-renewal.
We also found that Rs1-mediated Gs signaling in OBCs impaired BM erythropoiesis and megakaryopoiesis particularly after hematopoietic stress. However, Rs1 mice displayed normal erythropoiesis and megakaryopoiesis in the spleen and showed no overt signs of anemia or thrombocytopenia. It is therefore probable that the spleen, rather than the BM, produces the majority of circulating RBCs and platelets at steady state, whereas the BM defects only contribute to the delayed recovery observed after hematopoietic injury. These BM defects might result from erythroid/megakaryocyte progenitors being produced in lesser amounts than myeloid and lymphoid progenitors by the HSCs remaining in Rs1 BM or because they die upon differentiation because of poor support by the Rs1 BM microenvironment. Unfortunately, we could not detect Thrombopoietin (Tpo) or Erythropoietin (Epo) mRNA expression by quantitative RT-PCR in our isolated control or Rs1 OBCs (data not shown), hence limiting our ability to determine whether local production of these factors contributes to the observed BM defects. However, ELISA measurements on sera showed that, although Epo was unaffected, the level of circulating Tpo was significantly reduced in Rs1 mice (supplemental Figure 6). Although the mechanism leading to decreased Tpo production is unclear, it suggests that the hematopoietic defects in Rs1 mice may be influenced by systemic changes. We also observed a trend toward increased PTH levels in Rs1 serum (supplemental Figure 6), suggesting that secretion of endogenous hormones may also be indirectly affected. Together, these results indicate that continuous Gs activation in OBCs can induce broad systemic changes in key factors affecting the bone/hematopoietic axis and could contribute to lineage-specific defects in blood cell production.
Our findings raise the intriguing possibility that changes in Gs-GPCR signaling may regulate the development of particular blood cell lineages. Mice with impaired Gs signaling via deletion of the Gsα subunit in osteoprogenitors (GsαOsxKO mice) show decreased numbers of pro- and pre-B cell precursors, reduced levels of mature B cells in the BM and periphery, and attenuated production of IL-7.16 Our results indicate that the opposite (ie, Rs1-mediated increased Gs signaling in osteoblastic cells) neither affects B lymphopoiesis nor changes Il-7 expression (data not shown) but causes megakaryocyte and erythrocyte lineage defects that are exacerbated on hematopoietic injury. Several observations suggest that similar regulation might occur in humans. Patients with increased serum PTH levels, as in primary hyperparathyroidism, develop increased BM fibrosis, cortical bone loss, and maintained trabecular bone formation,39 yet generally show no anemia, except in rare cases of severe disease.40 In addition, patients with fibrous dysplasia, who have bone lesions resembling the Rs1 mice, sporadically show defects in their platelet number and function41 but not anemia (Michael Collins, written personal communication, February 18, 2012). Although Rs1 mice have significantly more bone disease than typically seen in fibrous dysplasia patients, our findings imply that a combination of bone disease burden and hematopoietic stressors may contribute to the rare cases of hematopoietic defects in hyperparathyroidism and fibrous dysplasia.
An important consideration for manipulating HSC function through GPCRs is that differences in Gs signaling induction may result in significantly different biologic effects. This appears to be the case for Cxcl12 mRNA levels, where exposure to a single PTH dose increases whereas continuous PTH stimulation decreases Cxcl12 expression in whole trabecular bone.42 CXCL12 protein levels are also increased in Opn+ cells in ColI(2.3)-caPTHR1 mice5 or by circadian oscillations of noradrenalin via the Gs-coupled β3-adrenergic receptor.43 In contrast, we observed decreased Cxcl12 mRNA levels in Rs1-expressing OBCs, which might be physiologic or the indirect consequence of ectopically expressing an engineered RASSL in OBCs. Nonetheless, these results illustrate that regulation of the hematopoietic maintenance and osteoblastic differentiation gene programs involves a complex regulatory network that responds differently to specific GPCRs (and their specific signaling cascades: ie, Gs, Gi, or Gq), specific ligands, and the temporal duration of receptor activation in individual osteoblast population subtypes. In particular, the Gs signaling pathway increases intracellular cAMP, which can transcriptionally regulate target genes via the cAMP response element binding proteins and protein kinase A.44 Several of the genes deregulated in Rs1-expressing OBCs, including Osx and Runx2 (osteoblast maturation) as well as Cxcl12 and Angpt1 (HSC maintenance), are directly regulated by cAMP.43,45,46 We also identified putative cAMP response element binding sites near the promoters of Cxcl12, Angpt1, VCAM1, SCF, Il-6, Runx2, OC, and Col1α1 genes using the CREB Target Gene Database47 (data not shown), suggesting that Gs-GPCR signaling can indeed coregulate bone differentiation and hematopoietic maintenance gene programs. Although additional studies are needed to fully understand the GPCR function in blood development, our results provide initial new insights into the molecular factors underlying the complex interplay between hematopoietic and stromal BM compartments.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Bruce Conklin, Robert Nissenson, Scott Kogan, Gary Howard, Jasmine Wong, Kevin Shannon, Trieu Nguyen, the Gladstone Histology Core, and the San Francisco Veterans Administration Bone Histomorphometry Core for valuable technical assistance and discussions.
This work was supported by the National Institutes of Health (K08 career development award AR056299), the National Osteoporosis Foundation (research grant, E.C.H.), and the National Institutes of Health (R01 grant HL092471, E.P.). K.S. was supported by The Netherlands Organization for Scientific Research (Rubicon grant) and the Dutch Cancer Society (fellowship grant). The J. David Gladstone Institutes mouse facility is supported by the National Center for Research Resources (grant RR18928-01).
National Institutes of Health
Authorship
Contribution: K.S., E.C.H., and E.P. conceived the project, designed the experiments, and wrote the manuscript; K.S. and E.C.H. carried out the experiments; T.G. and M.J.S. assisted with some of the experiments; and E.P. supervised the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Emmanuelle Passegué, Department of Medicine, Division of Hematology/Oncology, Eli and Edythe Broad Center of Regeneration Medicine and Stem Cell Research, University of California, San Francisco, 35 Medical Way, RMB 1017, San Francisco, CA 94143-0667; e-mail: passeguee@stemcell.ucsf.edu; or Edward Hsiao, Department of Medicine, Division of Endocrinology and Metabolism, University of California, San Francisco, 513 Parnassus Ave, S965, San Francisco, CA 94143-0794; e-mail: edward.hsiao@ucsf.edu.
References
Author notes
K.S. and E.C.H. contributed equally to this study.