Two articles in this issue of Blood from Feys et al and Callewaert et al, respectively, have employed very similar and elegant strategies in attempts to ameliorate the symptoms of thrombotic thrombocytopenic purpura (TTP).1,2
TTP is associated with severe deficiency in ADAMTS13, the metalloprotease that regulates von Willebrand factor (VWF) multimeric size and its platelet-tethering function.3,4 ADAMTS13 deficiency induces the persistence of pathogenic ultra-large (UL)–VWF in plasma that precipitates the formation of microvascular platelet-rich thrombi that variably cause microangiopathic haemolytic anaemia, thrombocytopenia, neurologic abnormalities, fever, and renal dysfunction. TTP is most frequently an acquired autoimmune disorder, arising through the formation of inhibitory antibodies against ADAMTS13. Untreated, the mortality rate of TTP is approximately 90%.
TTP is currently most effectively treated by plasma exchange, which serves to remove anti-ADAMTS13 antibodies and UL-VWF, and also replenish plasma ADAMTS13 activity. Steroids are used as immunosuppression to attain complete remission and, more recently, the use of rituximab has also proved highly efficacious in treating acquired TTP.5 Despite the appreciable reduction in mortality (to 10%-20%) using these therapeutic approaches, treatments are individualized and may be associated with further risks and complications. Therefore, new safer and simpler strategies to treat TTP are desirable.
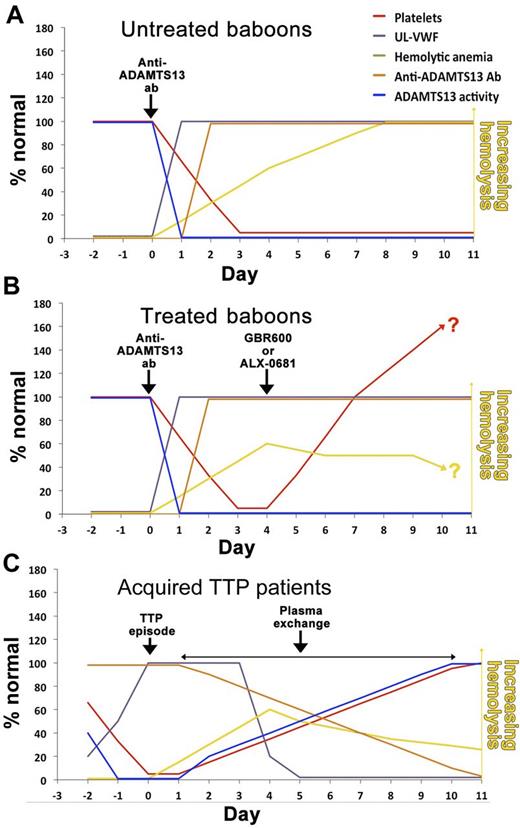
In mice, ADAMTS13 deficiency alone is insufficient to precipitate TTP-like symptoms, making it a difficult model in which to study novel therapeutic approaches.6 To circumvent this, and to better mimic the human scenario, Feys et al recently developed a baboon model of acquired TTP by infusing an anti-ADAMTS13 monoclonal antibody that efficiently inactivates plasma ADAMTS13.7 In baboons, this causes elevated plasma UL-VWF, platelet-rich thrombi in the microvasculature resulting in thrombocytopenia and haemolytic anaemia (see figure panel A). Although this nicely models early-stage human TTP, baboons do not develop life-threatening disease, or signs of neurologic or renal dysfunction. The clinical features of TTP are primarily linked to elevated plasma UL-VWF. For this reason, both Feys et al and Callewaert et al rationalized that targeting the glycoprotein Ib binding site in the VWF A1 domain might specifically prevent TTP.1,2
Schematic representation of parameters in the baboon model of TTP (A), with treatment with either GBR600 or ALX-0681 (B), or in patients with acquired TTP receiving plasma exchange therapy (C).
Schematic representation of parameters in the baboon model of TTP (A), with treatment with either GBR600 or ALX-0681 (B), or in patients with acquired TTP receiving plasma exchange therapy (C).
Feys et al employed a humanized mouse monoclonal antibody against the VWF A1 domain (termed GBR600), whereas Callewaert et al used a humanized bivalent nanobody recognising the same domain in VWF (termed ALX-0681). Both GBR600 and ALX-0681 avidly block VWF binding to glycoprotein Ib on the surface of platelets. Feys et al and Callewaert et al demonstrated that administration of GBR600 or ALX-0681, respectively, at the same time as the anti-ADAMTS13 antibody effectively inhibited UL-VWF function in baboons and prevented the onset of thrombocytopenia. Hemolytic anemia (as measured by the reduction in haptoglobin and the appearance of schistocytes) was also appreciably reduced using GBR600 or ALX-0681. Together these results demonstrated the UL-VWF-dependence of the TTP features seen.
These settings do not mirror the human situation where a patient presents with pre-existing symptoms. To model this, both studies allowed symptoms of TTP to develop for 4 days before administration of either GBR600 or ALX-0681. After treatment, both therapies caused a rapid normalization of platelet count after 3 days that continued to rise further over the following 3 to 4 days, suggesting that further platelet thrombi development and platelet consumption had been effectively inhibited (panel B). Improvement of hemolytic anemia was also evident through the gradual reduction in the numbers of schistocytes and signs of increases in plasma haptoglobin by the end of the 11-day study period. Crucially, there were no signs that blocking the VWF A1 domain caused appreciable risk of severe bleeding.
Together, these results are particularly encouraging and suggest that targeting VWF may be a highly effective strategy to prevent further development of symptoms in TTP. However, some important considerations remain in taking these studies further. The question arises as to whether blocking VWF is as effective as plasma exchange in ameliorating TTP in humans with more severe symptoms and, therefore, whether, in conjunction with immunosuppression, it has the potential to replace it. In acquired TTP patients, plasma exchange rapidly reduces antibody titer, UL-VWF and restores plasma ADAMTS13 activity to nonpathologic levels, enabling platelet counts to recover (panel C). It is likely that the restoration of ADAMTS13 activity by plasma exchange provides important therapeutic benefit for TTP patients that would be lacking with a VWF blocking approach alone, which allows the anti-ADAMTS13 antibodies and plasma UL-VWF to persist (panel B). Although both GBR600 and ALX-0681 showed signs of improvement of hemolytic anemia, Callewaert et al also showed that ALX-0681 did not alter the number of platelet-rich thrombi in treated baboons. Neither GBR600 nor ALX-0681 can dissociate preformed VWF-platelet complexes,1,2 which could be a limitation in treating TTP patients with more severe symptoms. Recently, Crescente et al demonstrated the thrombolytic potential of ADAMTS13 in a murine model of thrombosis, suggesting that ADAMTS13 activity might play an important role in the dissolution of platelet thrombi.8 Conceptually, this could be of particular benefit to TTP patients in ameliorating the effects of occluded vessels.
The 2 studies by Feys et al and Callewaert et al provide excellent evidence for the safety and efficacy of targeting VWF in TTP,1,2 which is also corroborated by some of the data using an aptamer (ARC1779) that similarly blocks the VWF A1 domain.9 However, the immune pathophysiology of TTP must still be treated separately. This is illustrated both by the tendency toward thrombocytopenia and hemolytic anemia after cessation of ALX-0681 in the baboons where the disease-inducing antibody 3H9 remains in circulation, and by data from the initial experience with ARC1779 in TTP patients.9 Whether it is feasible to employ the baboon model to make a comparison of GBR600 or ALX-0681 therapy with plasma exchange is unclear, but this could certainly enable the investigators to ascertain whether their therapeutic agents compare favorably with current treatment as well as defining whether plasma exchange does indeed carry important benefits associated with clearance of existing thrombi. This could determine whether VWF inhibition might in fact complement plasma exchange in TTP patients, in particular with respect to the time to platelet recovery and number of required plasma exchanges.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal