In this issue of Blood, Goussetis et al identify autophagy as a new pathway for the degradation of the oncoprotein BCR-ABL. They show that the therapeutic drug arsenic trioxide (AS2O3) targets BCR-ABL for autophagic degradation via a p62/SQSTM1-dependent mechanism that is critical for the antileukemic effect of the drug.1
Chronic myelogenous leukemia (CML) is characterized by the t(9;22) chromosomal translocation that juxtaposes the Breakpoint Cluster Region (BCR) gene to the Abelson (ABL) gene. The corresponding fusion protein BCR-ABL is endowed with a constitutive tyrosine kinase activity that is responsible for the occurrence of the disease. CML is paradigmatic in that it was the first leukemia to benefit from a targeted therapy with tyrosine kinase inhibitors (TKIs). TKIs have been shown to induce both apoptosis and autophagy in CML cells and the modulation of autophagy has recently emerged as a promising therapeutic option in CML and other leukemias.
Autophagy is a lysosomal catabolic process that is responsible for the turnover and elimination of damaged organelles and macromolecules. This process is used consistently by cells to promote their survival under adverse conditions such as stress signals and nutrient deprivation. Of note, autophagy can promote either cell survival or death, depending on the circumstances, making this process a promising target for therapeutic intervention. Indeed, autophagy participates in numerous biologic processes, such as cellular homeostasis, proliferation, differentiation, and cell death, all events that are potentially linked to leukemia progression and transformation.2
Using both pharmacologic and siRNA approaches, Goussetis et al describe the autophagic degradation of BCR-ABL by the lysosomal cysteine protease cathepsin B (CTSB) in CML cells treated with As2O3.1 They provide compelling evidence that p62/SQSTM1, a cargo protein involved in the targeting and elimination of ubiquitinated protein, is critical for the antileukemic effect of the drug. Clearly, these findings represent an important advance in our understanding of the regulation and function of BCR-ABL.
To date, several routes for the degradation of BCR-ABL have been reported including the proteasome,3 caspase-mediated degradation,4 CTSB-dependent cleavage consecutive to lysosomal membrane permeabilization,4 and autophagy.2 There is also evidence in the literature that autophagy is critical for the degradation of the PML-RARA (promyelocytic leukemia–retinoic acid receptor A) fusion protein.5,6 In both reports, the degradation of PML-RARA was associated with an increased antileukemic effect of As2O3, or all trans-retinoic acid (ATRA).
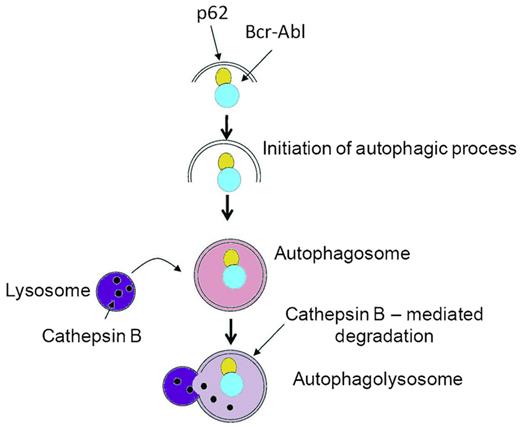
The results reported by Goussetis et al are important in several regards. First, this study identifies for the first time p62/SQSTM1 as a critical player in the autophagic degradation of the BCR-ABL fusion protein (see figure). p62/SQSTM1 is a versatile protein that exerts both pro and anti-tumor functions.7 Importantly, the study by Goussetis et al clearly identifies p62/SQTM1 with antileukemic properties in CML. In future experiments, it will be interesting to determine whether the interaction of p62/SQSTM1 with BCR-ABL is a direct one. Indeed, BCR-ABL has been shown to be ubiquitinated and p62/SQSTM1 is known to carry a ubiquitin-binding domain that could promote the direct interaction of both proteins.7 Second, from a mechanistic point of view, it will be important to decipher how As2O3 initiates the interaction of p62/SQSTM1 with BCR-ABL. Along this line, the phytoalexin resveratrol has been shown to promote the interaction of p62/SQSTM1 with microtubule-associated protein light chain 3 (LC3), thus favoring autophagy.8
p62/SQSTM1-dependent autophagic degradation of BCR-ABL in CML. Proposed model for the As2O3-dependent interaction of p62/SQSMT1 with BCR-ABL and subsequent autophagic degradation of BCR-ABL. The As2O3 treatment of CML cells triggers the interaction of p62 with BCR-ABL and the subsequent CTSB-mediated autophagic degradation of the fusion protein that is responsible for the antileukemic effect of the drug. The inhibition of autophagy by different means (pharmacologic agents or siRNA-mediated inhibition of Atg7, p62/SQSTM1, or CTSB) impairs BCR-ABL degradation and the effect of AS2O3, demonstrating the crucial role of autophagy in the effect of As2O3. Adapted from Figure 6 in the article by Goussetis et al that begins on page 3555.
p62/SQSTM1-dependent autophagic degradation of BCR-ABL in CML. Proposed model for the As2O3-dependent interaction of p62/SQSMT1 with BCR-ABL and subsequent autophagic degradation of BCR-ABL. The As2O3 treatment of CML cells triggers the interaction of p62 with BCR-ABL and the subsequent CTSB-mediated autophagic degradation of the fusion protein that is responsible for the antileukemic effect of the drug. The inhibition of autophagy by different means (pharmacologic agents or siRNA-mediated inhibition of Atg7, p62/SQSTM1, or CTSB) impairs BCR-ABL degradation and the effect of AS2O3, demonstrating the crucial role of autophagy in the effect of As2O3. Adapted from Figure 6 in the article by Goussetis et al that begins on page 3555.
The study by Goussetis et al paves the way for the possibility of new therapeutic intervention in CML. TKIs that target BCR-ABL are currently the leading compounds for patients suffering CML, leading to complete remission in a majority of cases. However, although they inhibit the tyrosine kinase activity of BCR-ABL, TKIs failed to eliminate the so-called leukemic initiating cells (LICs) that are critically involved in the reinitiation of the disease in a non-negligible proportion of the treated patients. Conversely to TKIs, As2O3, ATRA, or resveratrol, all drugs that are susceptible to target LICs could represent new therapeutic options in the treatment of CML. This should be achieved using combination of TKIs with the above-mentioned compounds.
Furthermore, BCR-ABL promotes the activation of the mammalian target of rapamycin (mTOR) pathway and as such acts as a potent inhibitor of autophagy, either directly or via inhibition of the adenosine monophosphate kinase. As2O3 and resveratrol are both capable to inhibit the mTOR pathway and to trigger CTSB-dependent BCR-ABL degradation.8,9 In the future, we must build on these dual effects of As2O3 for a better approach to CML treatment.
In conclusion, it is clear that the therapeutic modulation of autophagy represents a new avenue for the treatment of leukemia. The discovery that different clinically well-characterized therapeutic drugs trigger their effects via the autophagic degradation of oncogenic fusion proteins is of outstanding importance in oncohematology. Accordingly, the article by Goussetis et al sheds new light on the regulation and function of BCR-ABL, representing the premises for the use of these drugs in combination with TKIs in chronic phase CML and as a single therapy in TKI-resistant patients.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal