Abstract
Inflammatory cytokines and growth factors drive angiogenesis independently; however, their integrated role in pathologic and physiologic angiogenesis is not fully understood. Suppressor of cytokine signaling-3 (SOCS3) is an inducible negative feedback regulator of inflammation and growth factor signaling. In the present study, we show that SOCS3 curbs pathologic angiogenesis. Using a Cre/Lox system, we deleted SOCS3 in vessels and studied developmental and pathologic angiogenesis in murine models of oxygen-induced retinopathy and cancer. Conditional loss of SOCS3 leads to increased pathologic neovascularization, resulting in pronounced retinopathy and increased tumor size. In contrast, physiologic vascularization is not regulated by SOCS3. In vitro, SOCS3 knockdown increases proliferation and sprouting of endothelial cells costimulated with IGF-1 and TNFα via reduced feedback inhibition of the STAT3 and mTOR pathways. These results identify SOCS3 as a pivotal endogenous feedback inhibitor of pathologic angiogenesis and a potential therapeutic target acting at the converging crossroads of growth factor– and cytokine-induced vessel growth.
Introduction
Physiologic angiogenesis is tightly regulated. However, in proliferative retinopathy and cancer, there is excessive and disorganized growth of pathologic blood vessels. We hypothesized that there are endogenous angiostatic regulators that restrain pathologic vascular growth triggered by massive inflammatory and growth factor angiogenic stimuli. In the present study, we examined suppressor of cytokine signaling-3 (SOCS3) as such an endogenous angiostatic regulator.
SOCS proteins are known negative feedback regulators of inflammation and growth factor signaling.1,2 SOCS3 is transiently induced by inflammatory mediators such as lipopolysaccharide,3 IL-6,3 and TNFα.4 SOCS3 inhibits cytoplasmic effectors such as the JAK/STAT kinases and deactivates tyrosine kinase receptor signaling, including the IGF-1 receptor.1,5 It also regulates endothelial cell (EC) apoptosis.4 However, the role of SOCS3 in regulating angiogenesis in vivo is unknown. If SOCS3 were angiostatic, as has been hypothesized, then its suppression would increase neovascularization in pathologic conditions (see Figure 1A).
Systemic deletion of Socs3 is embryonically lethal.6,7 Therefore, in the present study, we generated Tie2-specific conditional Socs3 knock-out mice (Tie2-Socs3ko)8 to compare developmental and pathologic angiogenic responses with littermate controls (Socs3flox/flox). In oxygen-induced retinopathy (OIR),9-12 which mimics proliferative retinopathy, and in 2 tumor models, vascular deletion of Socs3 increased pathologic angiogenesis. Lack of SOCS3-mediated suppression of STAT3 and mTOR activation promoted EC proliferation and vascular sprouting. SOCS3 is therefore a newly identified endogenous negative regulator of angiogenesis acting on both inflammation and growth factor–mediated vessel formation specifically in pathologic contexts.
Methods
Animal models
Experiments adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals13 and were approved by the Boston Children's Hospital Animal Care and Use Committee. Tie2-Cre–expressing C57Bl/6 mice were crossed with Socs3flox/flox mice (Dr Yoshimura, Department of Microbiology and Immunology, Keio University School of Medicine, Tokyo, Japan14 ) to generate Tie2-Socs3ko and littermate control mice (Socs3flox/flox). In OIR, mice were exposed to 75% oxygen from P7-12. Retinas were dissected at the disease peak (postnatal day 17 [P17]) and neovascularization analyzed using SWIFT_NV.15 Mice < 5 g (P17) were excluded.11 For tumor implantations, mouse Lewis lung carcinoma (LLC; CRL-1642; ATCC) and B16F10 melanoma (CRL-6475; ATCC) cells were cultured as described previously.16 Male transgenic and control mice were injected subcutaneously with 1 × 105 LLC or B16F10 melanoma cells and tumor microvascular densities were quantified using computer-assisted morphometric analysis.17
Laser capture microdissection and quantitative RT-PCR
Eyes were enucleated and retinal layers were laser microdissected (Leica LMD6000). Isolated retinal RNA was reverse transcribed into cDNA for quantitative RT-PCR analysis with Cyclophilin A, TNFα, and Socs3 primers (see supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Immunohistochemistry and protein analysis
Retinas and tumors were fixed in 4% paraformaldehyde. Tumor vessels were stained for CD31 to measure vascular density.16 Retinal cross-sections stained for lectin and P-mTOR or P-STAT3 were imaged by epifluorescence and confocal microscopy. For Western blotting, retinas or human retinal ECs transfected with non-targeting (NT) or Socs3 siRNA (48 hours) were pretreated or not with TNFα (4 ng/mL for 15 minutes), stimulated with IGF-1 (50 ng/mL), snap-frozen, and lysed.
Microvascular sprouting from aortic explants and EC proliferation
Vascular sprouting experiments using aortic explants18 from Socs3flox/flox or Tie2-Socs3ko mice in basal serum and VEGF to ensure EC survival were stimulated with TNFα (4 ng/mL), IGF-1 (50 ng/mL), or both.
Statistical analysis
Results are presented as means ± SEM and were compared using a 2-tailed Student t test. P < .05 was considered statistically significant.
Results and discussion
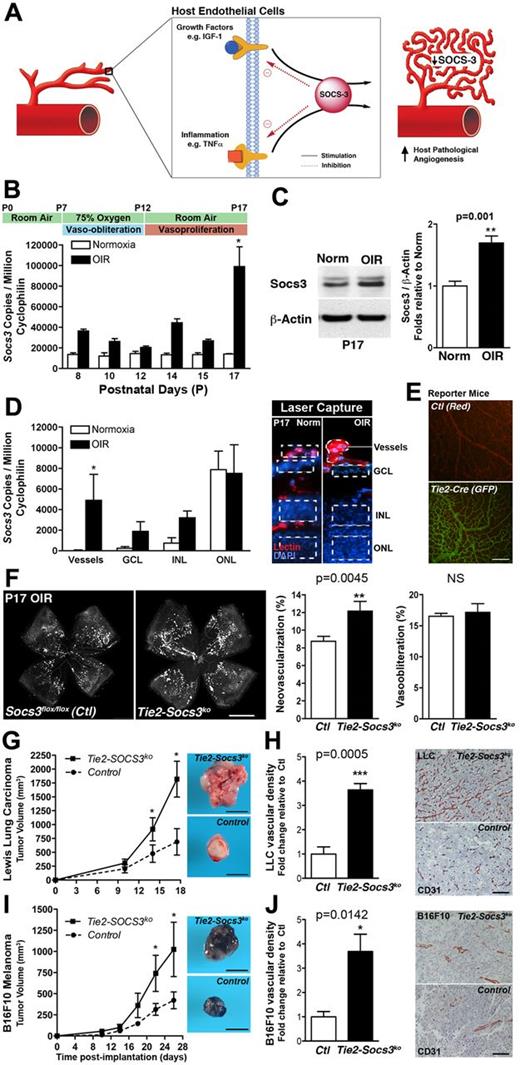
In OIR, hypoxia-induced pathologic angiogenesis peaks at P17,18 coinciding with increased TNFα (supplemental Figure 1A) and Socs3 expression (Figure 1B-C) and uncovering a temporal association between SOCS3 and pathologic retinal angiogenesis. To localize Socs3 expression in OIR mice, retinal layers and vessels were laser-capture microdissected (Figure 1D). The highest relative increase of Socs3 was found in pathologic retinal vessels (11-fold, P < .03, n = 4). Socs3 expression in the outer nuclear layer (photoreceptor cell nuclei), was unchanged in OIR compared with controls and therefore unlikely to contribute to pathologic angiogenesis. The ganglion cell layer and inner nuclear layer exhibited only slight increases in Socs3 expression with OIR. Endogenous SOCS3 is therefore highly and specifically induced in retinal blood vessels during pathologic angiogenesis.
SOCS3 attenuates pathologic neovascularization. (A) Schematic representation of SOCS3 functioning as a negative feedback modulator of angiogenic activation. (B-C) Socs3 expression in wild-type mice exposed to OIR compared with normoxia both at the mRNA and protein level (n = 3-4). (D) Socs3 expression is significantly up-regulated in pathologic neovessels obtained by laser microdissection of retinal layers (outlined on the right; n = 4). GCL indicates ganglion cell layer; INL, inner nuclear layer; and ONL, outer nuclear layer. (E) Retinal vasculature of Cre reporter mice Rosa mTflox/mG. This strain universally expresses a red-fluorescent protein (mT) that is removed in tissues with Cre recombinase expression, revealing instead a green fluorescent protein (mGFP). In control mice, the retinal vasculature is red (top), whereas in Tie2-Cre mice, the retinal vasculature is green (bottom), demonstrating that Tie2-Cre recombinase is expressed in retinal vessels (n = 3). (F) Conditional Tie2-Socs3ko mice have more pathologic neovascularization in OIR compared with Socs3flox/flox control mice. The area of vaso-obliteration is unchanged (n = 13-34). (G,I) Increased growth of LLC (G; n = 5-7) and B16F10 melanoma tumors (I; n = 5-9) in Tie2-Socs3ko compared with Socs3flox/flox mice. (H,J) Increased tumor vessel density of LLC (H; n = 5-7) and B16F10 melanoma tumors (J; n = 5-9) in Tie2-Socs3ko compared with Socs3flox/flox mice. Scale bar in panel E indicates 200 μm; panel F, 1 mm; panels G and I, 1 cm; and in panels H and J, 100 μm. *P < .05, **P < .01, P < .001.
SOCS3 attenuates pathologic neovascularization. (A) Schematic representation of SOCS3 functioning as a negative feedback modulator of angiogenic activation. (B-C) Socs3 expression in wild-type mice exposed to OIR compared with normoxia both at the mRNA and protein level (n = 3-4). (D) Socs3 expression is significantly up-regulated in pathologic neovessels obtained by laser microdissection of retinal layers (outlined on the right; n = 4). GCL indicates ganglion cell layer; INL, inner nuclear layer; and ONL, outer nuclear layer. (E) Retinal vasculature of Cre reporter mice Rosa mTflox/mG. This strain universally expresses a red-fluorescent protein (mT) that is removed in tissues with Cre recombinase expression, revealing instead a green fluorescent protein (mGFP). In control mice, the retinal vasculature is red (top), whereas in Tie2-Cre mice, the retinal vasculature is green (bottom), demonstrating that Tie2-Cre recombinase is expressed in retinal vessels (n = 3). (F) Conditional Tie2-Socs3ko mice have more pathologic neovascularization in OIR compared with Socs3flox/flox control mice. The area of vaso-obliteration is unchanged (n = 13-34). (G,I) Increased growth of LLC (G; n = 5-7) and B16F10 melanoma tumors (I; n = 5-9) in Tie2-Socs3ko compared with Socs3flox/flox mice. (H,J) Increased tumor vessel density of LLC (H; n = 5-7) and B16F10 melanoma tumors (J; n = 5-9) in Tie2-Socs3ko compared with Socs3flox/flox mice. Scale bar in panel E indicates 200 μm; panel F, 1 mm; panels G and I, 1 cm; and in panels H and J, 100 μm. *P < .05, **P < .01, P < .001.
To explore the role of OIR-induced Socs3 up-regulation in retinal vasculature, we created a Tie2-specific knockout of Socs3 (Tie2-Socs3ko). The expression of Tie2-Cre in vessels was confirmed with floxed reporter mice that changed from red to green fluorescence in Cre-expressing cells19 (Figure 1E). Conditional Tie2-Socs3ko mice subjected to OIR had more pathologic retinal neovascularization compared with littermate controls (Socs3flox/flox) at P17 (Figure 1F; Tie2-SOCS3ko, 12.15% ± 1.11%; Socs3flox/flox, 8.74% ± 0.56%; P = .0045, n = 13-34). The vaso-obliterated retinal area did not differ between Tie2-Socs3ko and Socs3flox/flox mice, suggesting that the vascular repair and regrowth of normal vessels was unchanged (Tie2-Socs3ko, 17.17% ± 1.36%; Socs3flox/flox, 16.52% ± 0.46%; P = .56). In addition, conditional Socs3ko and control mice had normal retinal vascular development (supplemental Figure 1B) and a similar rate of vascular dropout during the first phase of OIR (P7-P12; supplemental Figure 1C). These results indicate that SOCS3 expressed in the superficial retinal vascular layer plays a regulatory role in reducing angiogenesis under pathologic conditions while not affecting physiologic vascular development, vessel loss, or regrowth of normal vessels.
In the present study, we used 2 murine tumor models to confirm that SOCS3 regulates pathologic angiogenesis, a rate-limiting step of tumor size.20 LLC or B16F10 melanoma cells were injected subcutaneously into Tie2-Socs3ko and Socs3flox/flox controls. In both models, tumor volumes were significantly larger in Tie2-Socs3ko mice compared with controls (Figure 1G; LLC, 2.6-fold larger, P = .026, Figure 1I; B16F10, 2.4-fold larger, P = .044). As anticipated, the vascular density was increased in tumors implanted in Tie2-Socs3ko compared with control mice (Figure 1H; LLC, 3.6-fold, P = .0005, Figure 1J; B16F10, 3.7-fold, P = .014). Conditional Socs3 deletion was restricted to Tie2-expressing cells of the transgenic host. Expanding on previous findings that the loss of Socs3 in tumors was associated with a more aggressive phenotype,21 our present results highlight the contribution of the host environment and vessels in regulating tumor growth; tumor cells remained genetically unchanged in our models. Combined with the OIR results, our tumor data suggest that SOCS3 is an endogenous inhibitor of angiogenesis, the specific induction of which in pathologic settings serves to limit, but not completely abrogate, pathologic neovessel formation.
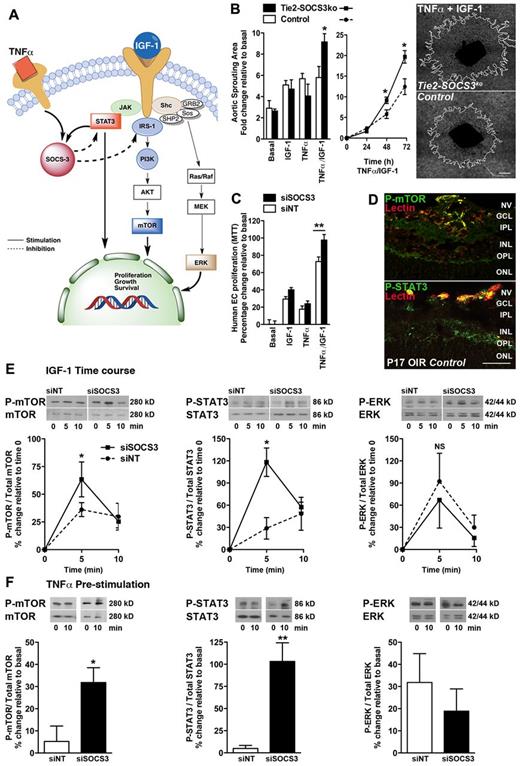
The SOCS3-dependent angiostatic mechanism was further explored ex vivo. During angiogenesis, EC activation is mediated by growth factors22 (eg, IGF-1 or VEGF) and inflammatory mediators23 (eg, TNFα or IL-6; Figure 2A). IGF-124,25 and TNFα26,27 signaling promote pathologic angiogenesis both in OIR and tumor growth, whereas inhibition of either pathway curtails pathologic angiogenesis. We therefore explored the role of SOCS3 as a central regulator of both pathways. Stimulation with either IGF-1 or TNFα alone did not induce a differential sprouting response, but together they increased vascular sprouting in Tie2-SOCS3ko aortic explants compared with controls (Figure 2B, P = .041). Similarly, SOCS3-silenced (siRNA) human retinal ECs (hRECs; supplemental Figure 2A) stimulated with both IGF-1 and TNFα showed increased proliferation (Figure 2C, P = .0085); these results were confirmed in mouse ECs (supplemental Figure 2B). Downstream of growth factor and cytokine signaling, both mTOR and STAT3 pathways are known inducers of SOCS3 and are activated in pathologic retinal angiogenesis28,29 (Figure 2D). We found increased activation of mTOR and STAT3 in IGF-1–stimulated primary hRECs (Figure 2E). Silencing SOCS3 (siRNA) further increased both mTOR and STAT3 phosphorylation, albeit briefly, compared with controls (5 minutes; 43%, P = .04 and 89%, P = .02), but not ERK signaling. However, prestimulation with TNFα followed by IGF-1 led to a sustained increase of both mTOR and STAT3 phosphorylation in SOCS3-silenced hRECs (Figure 2F, 27%; P = .02 and 98% above controls, 10 minutes; P = .01). Therefore, SOCS3 is an important antiangiogenic negative feedback regulator of pathologic angiogenesis acting at the crossroads of growth factor and cytokine-induced endothelial activation.
SOCS3 regulates vascular sprouting and endothelial activation. (A) Schematic representation of the signaling pathways regulated by SOCS3. (B) Increased sprouting of TNFα/IGF-1–stimulated aortic rings from Tie2-Socs3ko compared with Socs3flox/flox mice (n = 4-6). (C) Increased proliferation of human ECs treated with SOCS3 siRNA compared with controls (n = 9-10). (D) Expression of phospho-mTOR (P-mTOR) and P-STAT3 (green) in neovascular tufts (NV, red) of the OIR retina of Tie2-Socs3ko (not shown) and Socs3flox/flox control mice (n = 4). GCL indicates ganglion cell layer; INL, inner nuclear layer; and ONL, outer nuclear layer. (E) Increased mTOR and STAT3 phosphorylation, but not ERK, in IGF-1–stimulated ECs treated with Socs3 siRNA compared with controls (n = 3-5). (F) Prestimulation with TNFα (15 minutes) before IGF-1 further pronounces the increase of P-mTOR and P-STAT3 in IGF-1–stimulated ECs treated with Socs3 siRNA compared with controls (n = 3-6). Scale bar in panel B indicates 1 mm; and panel D, 50 μm. NS indicates not significant, *P < .05, **P < .01.
SOCS3 regulates vascular sprouting and endothelial activation. (A) Schematic representation of the signaling pathways regulated by SOCS3. (B) Increased sprouting of TNFα/IGF-1–stimulated aortic rings from Tie2-Socs3ko compared with Socs3flox/flox mice (n = 4-6). (C) Increased proliferation of human ECs treated with SOCS3 siRNA compared with controls (n = 9-10). (D) Expression of phospho-mTOR (P-mTOR) and P-STAT3 (green) in neovascular tufts (NV, red) of the OIR retina of Tie2-Socs3ko (not shown) and Socs3flox/flox control mice (n = 4). GCL indicates ganglion cell layer; INL, inner nuclear layer; and ONL, outer nuclear layer. (E) Increased mTOR and STAT3 phosphorylation, but not ERK, in IGF-1–stimulated ECs treated with Socs3 siRNA compared with controls (n = 3-5). (F) Prestimulation with TNFα (15 minutes) before IGF-1 further pronounces the increase of P-mTOR and P-STAT3 in IGF-1–stimulated ECs treated with Socs3 siRNA compared with controls (n = 3-6). Scale bar in panel B indicates 1 mm; and panel D, 50 μm. NS indicates not significant, *P < .05, **P < .01.
The results of the present study identify SOCS3 as a novel endogenous antiangiogenic regulator induced in vessels on very high angiogenic stimulation seen in pathologic states such as proliferative retinopathy and cancer. Developmental angiogenesis, in contrast, relies on the localized and well-orchestrated physiologic expression of growth factors acting below the induction threshold of SOCS3 and its inhibitory consequences on vascular growth.
SOCS3 is complementary to other suppressors of angiogenesis. Instead of neutralizing extracellular angiogenic factors or interfering with their receptors, SOCS3 in ECs dampens the response to converging downstream intracellular proliferative signals. Therefore, SOCS3 defines a new class of antiangiogenic modulators that are aimed at the integrated endothelial response to simultaneous angiogenic signals, rather than suppressing individual angiogenic factors that may differ between diseases or patients.
In summary, the results of the present study demonstrate a novel and specific role for SOCS3 in limiting pathologic angiogenesis in proliferative retinopathy and cancer. Using SOCS3 as a paradigm, novel and complementary therapeutic approaches may be developed to inhibit pathologic angiogenesis while at the same time sparing physiologic vascular development.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Yoshimura for the Socs3flox/flox mice.
This work was supported by the National Institutes of Health (EY017017, EY022275, EY017017-04S1, and P01 HD18655), a Research to Prevent Blindness Senior Investigator Award, an Alcon Research Institute Award, the Lowy Medical Foundation, the National Cancer Institute (grant R01CA148633 to D.P.), the Deutsche Forschungsgemeinschaft, Freifrau von Nauendorf Stiftung, Forschungskommission Freiburg, and Deutsche Ophthalmologische Gesellschaft (to A.S.), the Canadian Institutes of Health Research (to J.S.J. and P.S.) and Canada Research Chairs (to P.S.), the Children's Hospital Boston Ophthalmology Foundation, a Charles Hood Foundation Child Heath Research Award, and the Blind Childrens Center (to J.C.).
National Institutes of Health
Authorship
Contribution: A.S., J.-S.J., D.P., and L.E.H.S. conceived and designed the experiments; A.S., J.-S.J., A.M.J., C.J.H., D.T.P., C.G.H., M.R.S., N.M.K., R.J.D., E.R.G., E.B., and D.P. performed the experiments; J.C., P.S., and E.B. provided expert advice; A.S., J.-S.J., and L.E.H.S. wrote the manuscript; and all authors analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lois E. H. Smith, MD, PhD, Harvard Medical School, Children's Hospital Boston, 300 Longwood Ave, Boston MA 02115; e-mail: lois.smith@childrens.harvard.edu.
References
Author notes
A.S. and J.-S.J. contributed equally to this work.
D.P. and L.E.H.S. contributed equally to this work.