Abstract
The most common cause of treatment failure in childhood acute lymphoblastic leukemia (ALL) remains relapse, occurring in ∼ 15%-20% of patients. Survival of relapsed patients can be predicted by site of relapse, length of first complete remission, and immunophenotype of relapsed ALL. BM and early relapse (< 30 months from diagnosis), as well as T-ALL, are associated with worse prognosis than isolated extramedullary or late relapse (> 30 months from diagnosis). In addition, persistence of minimal residual disease (MRD) at the end of induction or consolidation therapy predicts poor outcome because children with detectable MRD are more likely to relapse than those in molecular remission, even after allogeneic hematopoietic stem cell transplantation. We offer hematopoietic stem cell transplantation to any child with high-risk features because these patients are virtually incurable with chemotherapy alone. By contrast, we treat children with first late BM relapse of B-cell precursor ALL and good clearance of MRD with a chemotherapy approach. We use both systemic and local treatment for extramedullary relapse, mainly represented by radiotherapy and, in case of testicular involvement, by orchiectomy. Innovative approaches, including new agents or strategies of immunotherapy, are under investigation in trials enrolling patients with resistant or more advanced disease.
Introduction
Currently, approximately 80%-85% of children with acute lymphoblastic leukemia (ALL) can be cured through the application of reliable prognostic factors permitting use of risk-oriented treatment protocols.1-4 The most common cause of treatment failure in pediatric ALL remains relapse: it occurs in approximately 15%-20% of patients, resulting in an incidence of approximately 0.7 of 100 000 children per year in Europe. Thus, relapsed ALL is the fourth most common childhood malignancy, and the number of children with ALL who experience treatment failure each year is similar to the number of children with newly diagnosed acute myeloid leukemia (AML) or rhabdomyosarcoma.5 With intensive combination chemotherapy and allogeneic hematopoietic stem cell transplantation (HSCT), 30%-50% of all children with relapsed ALL can be cured.6-12 Thus, most children still die despite aggressive chemo-radiotherapy approaches, including transplantation, and novel salvage regimens are needed.
Over the last decade, important biologic and clinical differences have been identified between leukemic cells at diagnosis and relapse, including the acquisition of new chromosomal abnormalities and gene mutations, and reduced responsiveness to chemotherapeutic agents.10,13 Moreover, reliable and sound criteria have been validated to identify subgroups of patients at higher risk of death because of leukemia progression, and the use of allogeneic HSCT has improved their outcome. Novel approaches include new formulations of existing chemotherapeutic agents, new antimetabolites and nucleoside analogs, monoclonal antibodies directed against leukemia-associated antigens, and molecularly targeted drugs.14,15 However, expedited development of drugs with high probability of activity in relapsed ALL has yet to be optimized.
In this article, we summarize the most recent knowledge on the pathophysiology of ALL recurrence, the criteria used for patient stratification, and the outcome reported by the most credited pediatric cooperative groups worldwide, discussing how we treat these patients and how novel therapies can be integrated into regimens for relapsed ALL.
Pathophysiology of relapse
Most ALL relapses occur during treatment or within the first 2 years after treatment completion, although relapses have been reported to occur even after 10 years from diagnosis.5,16 The majority of relapses occur in the BM, either in an isolated form or combined with involvement of another site, mainly CNS or the testes; isolated CNS or testicular relapse or, much less frequently, relapse involving other extramedullary sites may also occur.
Leukemia relapse represents the outgrowth of a clonal cell population not completely eliminated by treatment. Sophisticated analyses on rearrangements of immunoglobulin or T-cell receptor (TCR) genes typical of ALL clones at diagnosis and recurrence may help clarify the origin of leukemia recurrence. Indeed, each case of ALL harbors a unique rearrangement of immunoglobulin or TCR genes. Matched-pair analyses have detected immunoglobulin and TCR phenotypic shifts from diagnosis to relapse, but retrospective studies suggested that the relapsing clone is present in the initial specimen and persists in greater copy numbers than others, indicating selection of a preexisting drug-resistant clone.10 Although the relapsing clone may be undetectable after postinduction therapy, it can be initially evidenced in BM as minimal residual disease (MRD) and subsequently as overt disease recurrence. This has led to the hypothesis that many leukemia relapses may be the result of the selection of a relatively resistant clone already present at initial diagnosis rather than generation of a novel clone by mutation.10 By contrast, late relapses may represent de novo development of a second leukemia from a common premalignant clone. In this respect, very late relapses of TEL/AML1-positive ALL may represent a new event occurring in a quiescent leukemic cell that harbors an otherwise silent fusion gene and that has escaped eradication during initial therapy.17 Based on TCR gene rearrangement status, common clonal origins between diagnosis and relapse were also documented in the majority of BM T-ALL recurrences, which occur early in 90% of cases.18 In the few cases of late T-ALL relapses, TCR gene rearrangement sequences changed between diagnosis and relapse in more than one-third of cases, thus suggesting that these recurrences should be considered as a second T-ALL rather than a resurgence of the original clone.18
A more recent approach to address the issue of the origin of relapsed ALL is based on the use of genome-wide DNA copy number analysis on matched samples collected at diagnosis and relapse.13 In a seminal study, Mullighan et al demonstrated that the majority of relapse samples harbored at least some of the genomic copy number abnormalities present at diagnosis.13 More in detail, in only a minority of ALL cases (6%), the relapse clone represents the emergence of a genetically distinct and thus unrelated second leukemia.13 In the remaining cases, either there were no differences in copy number abnormalities between the diagnostic and relapse clones (8%), or relapse represented the clonal evolution of the diagnosis leukemic population (34%), or the relapse clone acquired new lesions, while retaining some, but not all, of the lesions found in the diagnostic sample (52%).13 Many of the genetic alterations that emerge in the predominant clone at relapse have been reported to involve genes implicated in treatment resistance, such as CDKN2A/B and IKZF1.19 Notably, a recent study on gene expression analysis revealed a signature of differentially expressed genes from diagnosis to relapse that varies in early (< 36 months) and late (> 36 months) ALL relapses.20
CNS relapse has been reported to occur in 3%-8% of patients.21 Leukemic cells may enter the CNS from the BM of the skull into the subarachnoid space via the bridging veins or the cerebrospinal fluid (CSF) via the choroid plexus, invade cerebral parenchyma via brain capillaries, or infiltrate the leptomeninges via bony lesions of the skull.21 Leukemia blasts can also disseminate through nerve roots, then invading the subarachnoid space through the neural foramina. Solid tumors made up of malignant cells (ie, chloromas) can enter the extradural space by extension along the intervertebral foramina. Finally, leukemia cells can be seeded either by CNS hemorrhage if the circulating blood contains blasts, or at time of the diagnostic lumbar puncture, when the latter is traumatic.21 The classification of CNS status widely adopted includes: CNS1, no detectable blast cells in CSF; CNS2, < 5 leukocytes/μL with detectable blast cells in a cytocentrifuged preparation of CSF; and CNS3, the presence of overt CNS leukemia, as previously defined (≥ 5 leukocytes/μL with identifiable blast cells, or the presence of cranial nerve palsies). Risk factors predicting CNS relapse include T-cell immunophenotype, hyperleukocytosis, high-risk genetic abnormalities, and the presence of leukemic cells in the CSF at the time of diagnosis.21 Traumatic lumbar punctures with blast present in the cytospin have been shown to negatively affect outcome of patients with newly diagnosed ALL22,23 and to be associated with hyperleukocytosis.22 Although in the past both CNS2 status and traumatic lumbar punctures at time of diagnosis were shown to be risk conditions for CNS ALL relapse,22-24 the use of more effective systemic and CNS-directed treatment in contemporary trials may greatly reduce or even eliminate their adverse prognostic effect, thus rendering intensification of treatment to reduce the risk of relapse not indicated, albeit additional doses of intrathecal methotrexate (MTX) during induction are used by some groups.21 It is noteworthy that recently published evidence suggests that submicroscopic involvement of the BM with leukemia is a frequent finding in patients with isolated CNS relapse.25
An isolated testicular relapse is defined as unilateral or bilateral testiculomegaly, with biopsy-proven testicular involvement in the absence of BM disease (eg, M1 status with < 5% blasts). The testes are conventionally considered as a sanctuary site, although the physiologic basis for a blood-testes barrier is not completely understood. Leukemia cells are generally found within the interstitial space, a compartment where MTX concentrations are lower than those in the serum.26 Although a true isolated extramedullary relapse in the testes may exist,27 evidence of BM involvement can be shown by sophisticated methods, such as PCR, in up to 91% of patients with a morphologically isolated testicular relapse.28
Risk stratification of relapsed ALL and the role of MRD
The most recent protocols for treatment of relapsed ALL include patient stratification according to the clinical characteristics at diagnosis and relapse, and provide different therapeutic options, as well as different indications for HSCT, in patients belonging to different risk subgroups. The site of relapse and duration of first complete remission (CR) have been shown to influence both event-free survival (EFS) and overall survival (OS) in childhood ALL. Indeed, isolated BM relapse carries the worst prognosis, with isolated CNS, isolated testicular or other extramedullary relapse having a better prognosis, and combined BM and extramedullary relapse being associated with an intermediate prognosis.10,29,30 In the Berlin-Frankfurt-Münster (BFM) stratification, very early relapses were considered those occurring < 18 months from diagnosis; early relapses were those occurring between 18 months and 30 months from remission, whereas late relapses occur ≥ 30 months from remission.31 The Children's Oncology Group (COG) stratification defines an early BM relapse as that occurring < 3 years from diagnosis and a later BM relapse as that occurring > 3 years after diagnosis. The BFM group also demonstrated that children with T-cell ALL BM relapses have a much worse prognosis than B-cell precursor (BCP)–ALL, irrespective of the time between diagnosis and recurrence.31 The adverse impact of T-cell phenotype on outcome was confirmed in both COG and Pediatric Oncology Group (POG) retrospective studies.9,32 ALL relapses have been classified by the BFM group into 4 different groups of risk (S1, S2, S3, and S4) according to site of relapse, time from diagnosis to relapse, and immune phenotype, documenting a prognostic significance of this stratification.12 Details on this stratification, as well as on frequency of different risk groups and their probability of treatment response, are reported in Table 1. Notably, none of the classifications used captures other adverse risk factors, such as MLL rearrangement, hypodiploidy (< 44 chromosomes), or BCR-ABL translocation, or considers genetic abnormalities characterizing patients with greater chance of rescue (ie, those with TEL-AML1 fusion transcript), and we consider this a significant limitation.33
BFM classification of relapsed childhood ALL
| Group (% of relapsed cases) . | Definition of relapse . | % of patients reaching CR, % . | OS at 5 y with chemotherapy, % . | OS at 5 y with HSCT, % . |
|---|---|---|---|---|
| S1 (5%) | 1. Late extramedullary relapses | 99 | 60-70 | Not used |
| S2 (55%) | 1. Early extramedullary relapses | 97 | 40 | 60 |
| 2. Very early extramedullary relapses | ||||
| 3. Non-T late BM relapses | ||||
| 4. Non-T combined early/late relapses | ||||
| S3 (15%) | 1. Non-T early BM relapses | 80-85 | < 5 | 30 |
| S4 (25%) | 1. Very early BM relapses | 70-75 | < 5 | 25 |
| 2. Very early combined relapses | ||||
| 3. T-phenotype bone marrow relapses |
| Group (% of relapsed cases) . | Definition of relapse . | % of patients reaching CR, % . | OS at 5 y with chemotherapy, % . | OS at 5 y with HSCT, % . |
|---|---|---|---|---|
| S1 (5%) | 1. Late extramedullary relapses | 99 | 60-70 | Not used |
| S2 (55%) | 1. Early extramedullary relapses | 97 | 40 | 60 |
| 2. Very early extramedullary relapses | ||||
| 3. Non-T late BM relapses | ||||
| 4. Non-T combined early/late relapses | ||||
| S3 (15%) | 1. Non-T early BM relapses | 80-85 | < 5 | 30 |
| S4 (25%) | 1. Very early BM relapses | 70-75 | < 5 | 25 |
| 2. Very early combined relapses | ||||
| 3. T-phenotype bone marrow relapses |
Very early relapse indicates < 18 months from diagnosis; early relapse, > 18 months from diagnosis but < 6 months from treatment discontinuation; and late relapse, > 6 months from treatment discontinuation.
Persistence of MRD, either evaluated with molecular techniques or through flow-cytometry, after induction/consolidation therapy (ie, after 5 and 12-13 weeks from the beginning of treatment for relapse) also influences prognosis in children with relapsed ALL.11,34-36 MRD can be detected at the 0.01% level in most patients with either method.37 Children with MRD levels < 1 × 10−3 or 1 × 10−4 have been shown to carry a lower risk of recurrence than patients with higher levels of MRD.11,34,36 The predictive value of MRD also in relation to HSCT for childhood relapsed ALL was reported in studies showing that molecular persistence of leukemia before the allograft heralded a high chance of disease recurrence.38-40
What we do for the treatment of relapsed ALL
Standard salvage regimens for relapsed ALL are still mostly based on different combinations of the same agents used in frontline therapy in various doses and schedules.6,10,33 Many groups adopt treatment strategies consisting of risk-adapted, alternating short-course multiagent systemic and intrathecal chemotherapy, in some cases together with cranial/craniospinal irradiation, and conventional maintenance therapy. Overall, remission can be achieved in > 70% of early relapses and in up to 96% of late BM relapses.6,10-12,29 Response rates cluster approximately 40% in second and subsequent relapse,5 although only 19 of 235 survivors (8%) have been reported among patients achieving third remission after a second BM relapse.41
Few randomized trials comparing different reinduction regimens in risk-stratified children with relapsed ALL have been conducted.11,29,42,43 A POG study in children with BCP-ALL showed higher reinduction rates with weekly rather than biweekly pegylated asparaginase.42 A correlation between increased asparaginase levels and improved CR rate was shown.42 The BFM group randomized dose and duration of infusional MTX in reinduction, demonstrating similar outcomes between intermediate-dose (1 g/m2 over 36 hours) and high-dose (5 g/m2 over 24 hours) infusions and have adopted the former for future trials.29 Recently, the United Kingdom Children's Cancer Group enrolled in a trial 239 children with first ALL relapse, stratified into high-risk (HR), intermediate-risk (IR), and standard-risk (SR) groups on the basis of duration of first CR, site of relapse, and immunophenotype.11 Patients were allocated to receive either idarubicin or mitoxantrone during induction. After 3 blocks of therapy, HR and IR patients with a MRD ≥ 10−4 cells received allogeneic HSCT, whereas SR and IR patients with a MRD < 10−4 continued chemotherapy. Progression-free survival (PFS) and OS were significantly higher in the mitoxantrone group, and the differences in PFS were mainly related to a decrease in disease events (progression, second relapse, disease-related deaths). The 3-year OS was 69% in the mitoxantrone group (45% in the idarubicin), which overall represented a substantial improvement over preceding trials from the same investigators.11 This study suggests that, while novel and targeted therapeutic strategies are being developed, a conventional agent, such as mitoxantrone, can confer a significant benefit in children with relapsed ALL.11 In the ALL-REZ BFM 90 trial, 525 patients stratified into risk groups were enrolled and received alternating short courses of intensive polychemotherapy (blocks R1, R2, or R3) and cranial/craniospinal irradiation followed by maintenance therapy.6 Multiagent chemotherapy blocks R1 and R2, deriving from ancestor BFM studies,12 included 36-hour infusion of MTX, combined with other cytotoxic drugs. Block R3 (including high-dose cytarabine and etoposide) was introduced to improve the outcome compared with historical controls. The probabilities of EFS and OS at 10 years were 30% and 36%, respectively, similar to those of previous studies from the same Cooperative Group.29,43 Therefore, neither the introduction of block R3 nor the adaptation of chemotherapy intensity improved patient outcome.
In a recent COG report, postrelapse OS was evaluated in 272 patients who had been previously randomly allocated to initial treatment with augmented- or standard-intensity regimen of postinduction intensification.44 As expected, PFS was worse for early versus late relapse, marrow versus extramedullary site, adolescent versus younger age, and T-ALL versus BCP-ALL; however, no difference in 3-year OS was found for children given augmented- versus standard-intensity postinduction intensification.44
The Italian Pediatric Hematology Oncology Association has shown efficacy of high-dose idarubicin (40 mg/m2) combined with high-dose cytarabine in patients with HR relapsed ALL.45 However, idarubicin was not found to be superior to daunorubicin when used at lower dosage (10-12.5 mg/m2 per week for 3 administrations) in first BM relapse, as suggested by a Children's Cancer Group study.46
Given the paucity of randomized clinical trials, it remains unclear whether any reinduction combination in use today is significantly superior to any other. In our view, children with first BM relapse of ALL should be treated with chemotherapy protocols proved to be effective in reinducing and consolidating a second morphologic CR.6,11,44 The algorithm in Figure 1 gives an overview on how we approach management of a patient with first relapse of ALL. We stratify children with relapsed ALL into different risk groups for which diversified protocols are used. In particular, we treat HR children with more aggressive chemotherapy schemes.34,45,47 In any case, we recommend that relapsed ALL children be included in prospective, controlled trials. In this regard, the International Cooperative Group on Relapsed ALL will conduct 2 randomized trials comparing the classic BFM reinduction therapy with that reported by the United Kingdom Children's Cancer Group in SR/IR patients, and with a novel regimen combining clofarabine, etoposide, and cyclophosphamide in HR children, respectively. We use MRD measurements to allocate those SR/IR patients with poor blast clearance (∼ 50% of this subgroup) to allogeneic HSCT. In particular, we offer transplantation to SR/IR patients with MRD level > 1 × 10−4 (see also Figure 1). Outcome of patients not responding to reinduction treatment has been reported to be dismal.48 Further therapies with curative intent can induce CR2, but they are associated with high treatment-related morbidity, mortality, and minimal survival. Although treatment of these patients has been heterogeneous and customized, we think that they are candidates for controlled phase 1 or 2 trials (see “How can we integrate the most promising novel drugs into current regimens for relapsed ALL”), and we propose them enrollment in experimental studies both for offering a chance to benefit from new drugs and for fostering drug development for cohorts with better prognosis.
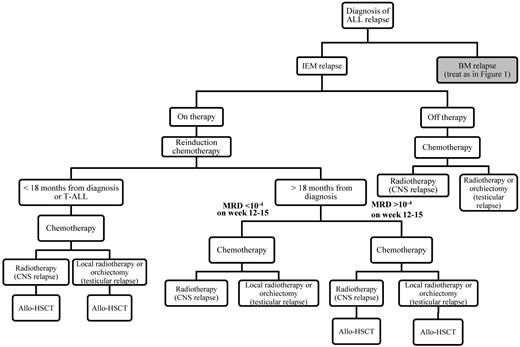
Algorithm that we follow for tailoring treatment of children with BM relapse of ALL according to the length of CR1, the immunophenotype of ALL, the presence of cytogenetic abnormalities, and the level of MRD after induction/consolidation therapy. IEM indicates isolated extramedullary relapse.
Algorithm that we follow for tailoring treatment of children with BM relapse of ALL according to the length of CR1, the immunophenotype of ALL, the presence of cytogenetic abnormalities, and the level of MRD after induction/consolidation therapy. IEM indicates isolated extramedullary relapse.
CNS relapse
Radiotherapy together with systemic chemotherapy is universally used for patients experiencing CNS recurrence (Figure 2). The efficacy of this approach has been demonstrated by several studies. In detail, the St Jude's Group reported in 20 children that an intensive retrieval therapy for isolated ALL CNS relapse, consisting of a 5-drug reinduction chemotherapy followed by 4 rotating drug pairs, triple intrathecal therapy, and then craniospinal irradiation, led to a second CR in all treated patients.49 In a POG study, BM relapse in patients with isolated CNS relapse has also been shown to be prevented by administering intensive chemotherapy before delayed craniospinal radiation.50 In this trial, 83 patients with ALL received systemic and intrathecal chemotherapy for 6 months, followed by craniospinal radiation (24 Gy cranial/15 Gy spinal) and by maintenance treatment. The 4-year EFS for patients with CR1 lasting > or < 18 months before CNS relapse was 83% and 46%, respectively.50 Reduction of the radiation dose to 18 Gy in a subsequent POG trial in 71 patients with BCP-ALL whose initial remission lasted > 18 months translated into an overall 4-year EFS rate of 78%.51 Conversely, patients with CR1 of < 18 months duration had a 4-year EFS of 52%. The BFM group reported a 54% probability of EFS of patients with isolated extramedullary relapse, regardless of time point of relapse.6 Together, these data suggest that, whereas the chance of rescuing patients with late ALL CNS relapse is high, the outcome of children experiencing early CNS recurrence has to be improved, perhaps also through the use of allogeneic HSCT (see “To whom and when we offer allogeneic HSCT in relapsed ALL”). Local radiotherapy is delivered after that intensive systemic therapy has been administered, with the aim of preventing/treating BM seeding of the leukemia cells (see also Figure 2), this strategy being supported also by the observation that submicroscopic leukemia BM involvement is a frequent finding. With the use of contemporary systemic therapy, there is no clear evidence of the superiority of craniospinal irradiation over cranial radiotherapy; we use for all patients with CNS involvement a radiation dose of 18 Gy, which is reduced to 15 Gy in case of prior cranial irradiation (> 20 Gy).
Algorithm that we follow for treatment of children with isolated extramedullary relapse (IEM) according to the length of CR1, the immunophenotype of ALL, and the level of MRD after induction/consolidation therapy.
Algorithm that we follow for treatment of children with isolated extramedullary relapse (IEM) according to the length of CR1, the immunophenotype of ALL, and the level of MRD after induction/consolidation therapy.
Testicular relapse
Either orchiectomy or irradiation has been used as local treatment for children with recurrence of ALL in the testes.9,27,52 There are no data supporting one approach over the other, although, in principle, orchiectomy may provide a greater chance for definitive eradication of testicular disease. We use orchiectomy in case of monolateral testicular involvement; we perform biopsy of the controlateral testis for demonstrating the absence of leukemia localization before administering prophylactic radiotherapy (15 Gy). In case of bilateral testicular localization, we consider either orchiectomy or radiotherapy (24 Gy) as appropriate (Figure 2). If there is no doubt that orchiectomy leads to infertility and abrogates sex hormone production, it has to be underlined that also local radiotherapy at the dosage used for bilateral testicular recurrence is expected to induce infertility and significantly impair hormone production.53
To whom and when we offer allogeneic HSCT in relapsed ALL
Allogeneic HSCT is frequently used for pediatric patients with ALL in CR2 after marrow relapse.6,31,32,40,54-56 Several studies have documented that allogeneic HSCT from matched family donors offers high chances of rescuing these patients, and the EFS has been reported to be superior to that achieved with second-line chemotherapy treatment.32,55 The BFM ALL-REZ-87 trial indicated that EFS was better after transplantation than after chemotherapy group only in patients with HR or IR ALL relapse.29 More recently, Eapen et al demonstrated an advantage of allogeneic HSCT only in children relapsing within 36 months from diagnosis when they receive a total body irradiation-based conditioning regimen.57 By contrast, the COG study CCG-1941 failed to demonstrate an advantage for patients given HSCT over those treated with chemotherapy only.8
More than 70% of children with relapsed ALL who might benefit from an allograft lack an HLA-identical family donor. With the establishment of donor registries, many patients are able to locate a suitable unrelated donor (UD). Results of relapsed ALL patients recently transplanted suggest that, in terms of ultimate outcome, using UD selected through high-resolution typing of HLA loci offers minimal or possibly no significant disadvantage compared with using an HLA-identical sibling.54,56,58 A matched-pair analysis from the BFM group comparing UD-HSCT with chemotherapy for children with ALL in CR2 documented that the probability of EFS was significantly higher for HR (44% vs 0%), but not IR (39% vs 49%) patients given the allograft.31
Cord blood has significantly extended the opportunity to perform HSCT to patients lacking an HLA-matched donor. Published reports have compared the outcome of umbilical cord blood transplantation (UCBT) and UD-BMT in children with hematologic malignancies.59-61 The Eurocord group published a study comparing the outcome of matched unrelated BMT (HLA 6 of 6 matched), either unmanipulated or T cell–depleted, with HLA-mismatched UCBT.59 The incidence of relapse was the same in the 3 groups analyzed, and the probability of EFS was comparable. Eapen et al compared results observed in 503 children given UCBT with those of 282 UD-BMT recipients (116 were HLA allele-matched [HLA-A, -B, -C and DRB1; thus, 8 of 8] and 166 mismatched).60 Of the UCBT recipients, only 35 were matched at the HLA A, B (antigen level) and DRB1 (allele level). In comparison with children given allele-matched UD-BMT, patients transplanted with 1 or 2 HLA-disparate, high-cell content UCB units had a similar 5-year EFS, whereas an even better outcome was evident for the 35 children given HLA-matched UCBT.60 These results have been more recently confirmed by Cousten-Smith et al, who analyzed 87 ALL CR2 children transplanted in a single institution from either HLA-matched siblings, or unrelated donors or with UCB.61 Ruggeri et al have recently reported on 170 children with ALL in CR; for the 77 transplanted in CR2, the 4-year EFS was 44% and high levels of MRD before the allograft predicted an increased relapse risk.40
T cell–depleted HSCT from an HLA-haploidentical relative (haplo-HSCT) offers an immediate transplant option for patients lacking a matched donor or a suitable cord blood unit.62 The absence of the T cell–mediated graft-versus-leukemia effect has also been thought to render the recipients of a T cell–depleted allograft more susceptible to leukemia relapse.62,63 However, fundamental studies on haplo-HSCT have shown that, in adult patients with AML transplanted in CR, a graft-versus-leukemia effect could be mediated by NK cells when an HLA-disparate, NK alloreactive relative was used as donor.64 This NK-mediated graft-versus-leukemia effect has also been documented in children with ALL.63,65 The Pediatric Diseases and the Acute Leukemia Working Parties of the European Blood and Marrow Transplant registry have analyzed outcomes of 127 children with ALL given haplo-HSCT. Whereas the EFS of children transplanted not in remission was 0%, that of children with CR2 and CR3 was 34% and 22%, respectively.66 Therefore, a T cell–depleted haplo-HSCT should be included in the treatment algorithm as a valuable option for patients with ALL in need of transplantation and lacking a matched donor, especially if an NK alloreactive relative exists. An unmanipulated HLA-haploidentical HSCT can be considered for those few patients who are unable to locate an HLA-compatible donor, a suitable UCB unit, or an NK-alloreactive relative.
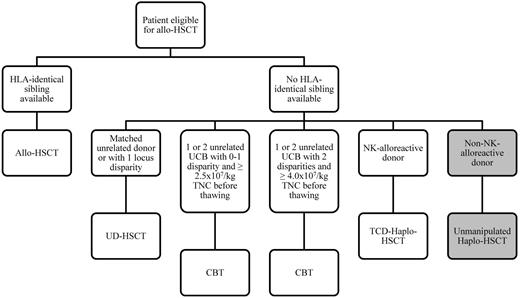
In our daily practice, we offer allogeneic HSCT to any child with either HR features or poor clearance of blast cells (eg, high levels of MRD) because these patients (approximately two-thirds of the overall population of relapsed children) have a probability of survival < 30% without transplantation (Figure 3). We still consider HLA-matched siblings as preferred donors; for children lacking an HLA-compatible family donor, either UD or UCB units or haploidentical family donor are suitable options. We use high-resolution molecular typing for HLA-A, -B, -C, -DRB1 loci for selecting unrelated BM donors, which should not differ from the recipient for more than 1 allele. Details on what we recommend in terms of cell content and characteristics of UCB units to be used are summarized in Figure 3. HLA-haploidentical donors, optimally if NK-alloreactive, can represent a further option in centers running specific programs. Whatever the type of transplant given, we perform HSCT in children achieving second CR after 2 or 3 courses of consolidation chemotherapy aimed at obtaining low MRD level because this portends favorable implications for disease recurrence.38-40 Although total body irradiation still represents the “golden standard,” alternative chemotherapy-based regimens should be tested in future controlled trials.
Algorithm that we follow for choosing the best transplant option for any child with relapsed ALL and an indication to allogeneic HSCT. The grey boxes represent what we consider to be a still experimental approach of unmanipulated, HLA-haploidentical HSCT. TCD indicates T-cell depleted; UCB, umbilical cord blood; CBT, cord blood transplantation; and TNC, total nucleated cells.
Algorithm that we follow for choosing the best transplant option for any child with relapsed ALL and an indication to allogeneic HSCT. The grey boxes represent what we consider to be a still experimental approach of unmanipulated, HLA-haploidentical HSCT. TCD indicates T-cell depleted; UCB, umbilical cord blood; CBT, cord blood transplantation; and TNC, total nucleated cells.
How can we integrate the most promising novel drugs into current regimens for relapsed ALL
After many years in which the portfolio of agents effective against ALL blasts has remained unchanged, the last decade has witnessed the development of novel pharmacologic agents, including nucleoside analogues, monoclonal antibodies, and new formulations of existing chemotherapeutic agents and targeted molecules, such as tyrosine kinase inhibitors and FMS-related tyrosine kinase 3 (FLT3) inhibitors.15 The creation in the United States of a Pediatric Preclinical Testing Program, a large inventory of pediatric tumor cell lines, preclinical human xenograft models and samples from patients, and the founding in Europe of the Innovative Treatment of Childhood Cancer consortium speak to the level of interest that exists for the development of new strategies. This said, it should be noted that many patients experiencing ALL relapse respond to the same drugs used during first-line treatment. Therefore, investigational agents are not generally used until patients experience subsequent relapse and become refractory to multiple therapies. We think that development of strategies for accelerating the evaluation and clinical development of novel agents is a priority.
Nucleoside analogs
Clofarabine is a second-generation purine analog capable of inhibiting DNA synthesis/repair and inducing cell death.67-70 In phase 1 ore 2 clinical trials, clofarabine was shown to display significant activity as a single agent in heavily pretreated children with refractory or relapsed ALL, with limited liver toxicity and no neurotoxicity.67,71 Treatment has also been associated with profound and long-lasting myelosuppression, with the maximum tolerated dose as single-agent therapy being 52 mg/m2 per day for 5 consecutive days.67,71 Clofarabine has been granted accelerated approval both in Europe and in the United States for the treatment of pediatric patients with relapsed or refractory ALL who received at least 2 prior regimens of chemotherapy. More recently, clofarabine has been tested in combination with agents expected to have a synergistic effect (cyclophosphamide and etoposide).47,72-74 In these combination studies, clofarabine was used at a maximum dosage of 40 mg/m2 per day for 5 consecutive days. In all these studies, the probability of reaching CR or CR without platelet recovery was remarkable (ie, 40%-60%).47,72-74 Importantly, these schemes had an acceptable safety profile, although adverse events were reported, including fatal infections and cases of veno-occlusive disease of the liver, especially in previously transplanted patients. The United Kingdom experience with clofarabine-based regimens for relapsed pediatric ALL formally demonstrated that the probability of response is higher in combination regimens than for single-agent use.74 In the same trial, clofarabine-based regimens were more effective when given in first relapse, with a CR rate of 86%, compared with 40% and 20% when given in second and third relapse, respectively.74 Responses were observed in all age groups and in children with adverse cytogenetics, namely, MLL rearrangement or a Ph chromosome.47,72-74 In 2 of the combination studies, the probability of CR was reported to be significantly higher in patients with BCP-ALL than with T-cell ALL.47,72 In view of this latter finding, we consider the use of clofarabine-based regimens in children with either resistant or second or subsequent BM relapse of BCP-ALL.
Nelarabine is an inhibitor of purine nucleoside phosphorylase; in a phase 1 study, it was given as a consecutive 5-day schedule inducing remarkable responses in patients with T-lineage ALL (54% of CR or partial response after 1 or 2 courses).75 In a phase 2 study restricted to children with refractory T-cell ALL, nelarabine at 1200 mg/m2 for 5 consecutive days induced a 35% CR rate in the 79 patients without CNS involvement.76 A higher rate was achieved in patients who were in first relapse (46%), compared with those in second relapse (25%) or with CNS disease (21%). The FDA approved nelarabine in October 2005 for the third-line treatment of patients with T-cell ALL/lymphoma.77 Recently, the COG AALL00P2 trial, a 2-stage pilot study, assessed the feasibility and safety of adding nelarabine to an intensive modified BFM 86 chemotherapy regimen in children with newly diagnosed, high-risk T-ALL. This study showed that nelarabine is well tolerated and gives encouraging results in pediatric patients with T-ALL, particularly those with a slow early response, who historically have a dismal prognosis.78 Thus, we think that combination schemes, including nelarabine, have to be implemented and tested for children with relapsed T-cell ALL.
Monoclonal antibodies
Epratuzumab is a humanized monoclonal antibody targeting the CD22 antigen, highly expressed in the majority of BCP-ALL.79 This compound has been used both in a window therapy and in combination with standard reinduction chemotherapy, in a pilot study enrolling 15 pediatric patients affected by BCP-ALL with BM relapse.80 Nine of these 15 patients achieved CR, 7 with negativity of MRD.80 The efficacy of epratuzumab will be further tested in a randomized trial being conducted by the International Cooperative Group on Relapsed ALL group and aimed at evaluating its role in reducing MRD levels in SR patients, thus decreasing the number of children eligible for HSCT. Anti-CD22 immunotoxins are under development to improve the efficacy of this antibody.
Blinatumomab is a murine, single-chain antibody construct belonging to a new class of bi-specific T-cell engagers and designed to link CD19-expressing B cells and T cells. This synapsis results in cytotoxic T-cell responses against CD19-expressing cells.81,82 In vitro data indicated that CD19+ lymphoma and leukemia cell lines are extremely sensitive to blinatumomab-mediated cytotoxicity.81,82
A phase 1 study, conducted in 38 adults with either relapsed or refractory B cell non-Hodgkin lymphoma, has demonstrated a dose-dependent efficacy of blinatumomab.83 The drug was effective in killing lymphoma cells not only in lymphoid organs, but also in the BM.
The German Multicenter Study Group on Adult ALL evaluated the safety and efficacy of blinatumomab in 21 adults with BCP-ALL and MRD persistence/relapse after induction and consolidation therapy.84 Patients received a 28-day continuous intravenous infusion of blinatumomab at 15 μg/m2 per day followed by a treatment-free period of 2 weeks. Responders either received 3 additional consolidation cycles of blinatumomab or were given HSCT. Sixteen patients became MRD negative. Remarkably, 12 of them had been molecularly refractory to previous chemotherapy.84 This study suggests that blinatumomab has the potential to induce, as a single agent, durable remissions in molecularly refractory BCP-ALL.
More recently, 18 adult patients with relapsed/refractory BCP-ALL were given blinatumomab in an exploratory phase 2 trial.85 The drug was administered by continuous infusion for 28 days followed by a 14-day treatment-free interval at 3 dose levels of 5-30 μg/m2 per day. Twelve of them (67%) reached CR within the first 2 cycles of treatment. Of these 12 responding patients, 75% had complete hematologic recovery and all reached MRD negativity within the first 2 cycles. This study indicates that the drug is also effective in ALL adults with either overt relapse or hematologic resistance to conventional treatment.
Available data on the safety and efficacy of blinatumomab in children are much more limited. Handgretinger et al reported on 3 pediatric patients with ALL who relapsed after allogeneic HSCT.86 Children were given the drug at 15 μg/m2 per day for at least 4 weeks. All patients showed molecular CR after 4 weeks of treatment. Safety was described as acceptable, without treatment interruptions or discontinuations because of adverse events.86 Further studies on the drug in relapsed ALL are ongoing.
Bortezomib is a proteasome inhibitor, which renders leukemic cells more sensitive to the apoptotic effects of chemotherapy. Bortezomib is synergistic with dexamethasone and additive with cytarabine, doxorubicin, asparaginase, and vincristine.14 The efficacy of bortezomib with cytotoxic drugs used for reinducing remission in ALL patients (ie, dexamethasone, vincristine, pegylated asparaginase, and doxorubicin) has been recently reported in a phase 1 study.87 An extended phase 2 study enrolling 22 children was recently reported by the Therapeutic Advances in Childhood Leukemia consortium. All patients had failed 2 or 3 prior regimens; CR and CR without platelet recovery were obtained in 73% of patients, and this percentage increased to 80% in BCP-ALL.88 These results led to a premature discontinuation of the trial for evident superiority with respect to historical results.
Targeted molecular treatments are a novel and promising approach in the management of ALL. The most successful example of molecularly targeted therapy in ALL is that of the tyrosine kinase inhibitors. Imatinib mesylate proved to be effective in improving the outcome of childhood Philadelphia (Ph)–positive ALL,89 and it can certainly be used in the context of relapsed ALL, provided that molecular studies on relapsed samples do not show mutations conferring resistance to the drug. Dasatinib and nilotinib have been shown to be active and efficacious in imatinib-resistant Ph+ malignancies and dasatinib is replacing imatinib in several Ph+ ALL regimens for adults.90,91 The use of these novel tyrosine kinase inhibitors, both in reinduction and for preventing leukemia recurrence after an allograft, remains to be tested in future trials.
FLT3 is frequently overexpressed in MLL-rearranged ALL, in c-kit (CD117)–positive T-cell ALL and in hyperdiploid ALL. FLT3 inhibitors display in vitro cytotoxicity in samples of BCP-ALL with MLL rearrangements and activated FLT3.92,93 A multicenter trial on MLL-rearranged ALL in children older than 1 year is ongoing.
Liposomal cytarabine, a sustained-release formulation of cytarabine encapsulated into multivesicular lipid-based particles, administered intrathecally every 2 weeks, demonstrated greater efficacy in pediatric patients with neoplastic meningitis compared with more frequent administration of free cytarabine or MTX.94,95 However, serious neurotoxicity has been reported when using intrathecal liposomal cytarabine in association with high-dose MTX.96 Although further studies are needed to define the role of liposomal cytarabine in relapsed ALL with CNS involvement and resistance to conventional therapy, this drug could become the agent of choice for clearing resistant CNS disease.
We recommend that any child with second relapse of ALL or refractory disease be included into experimental trials aimed at testing/validating the safety and efficacy of novel agents. More specifically, whenever feasible, each child with ALL in second relapse or resistant to either first-line or second-line therapy treated at our institution is enrolled in phase 1 or 2 trials with one of the drugs discussed earlier in this section. Indeed, we think that the development of these novel approaches holds great promise for the future, and it is desirable that the most successful of these compounds be soon incorporated into the first-line treatment of patients with HR relapsed ALL.
Acknowledgments
This work was supported in part by Associazione Italiana Ricerca sul Cancro (IG #8556, S.R.; and Special Grant “5 per mille,” F.L.), Ministero dell'Istruzione, Università e Ricerca Scientifica (F.L.), Istituto di Ricovero e Cura a Carattere Scientifico Bambino Gesù Children's Hospital, Rome (Progetto di Ricerca Corrente), and European Community FP7 (Project 278514, F.L.).
Authorship
Contribution: F.L., M.S., M.E.B., and S.R. designed the study and wrote, edited, and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Franco Locatelli, University of Pavia, Istituto di Ricovero e Cura a Carattere Scientifico Bambino Gesù Children's Hospital, Piazzale Sant'Onofrio, 4, 00165 Rome, Italy; e-mail: f.locatelli@opbg.net.