Abstract
Interleukin-7 (IL-7) is a nonredundant cytokine that plays a critical role in T-cell homeostasis and promotes immunologic reconstitution in lymphopenic hosts. Here, we show that IL-7, at doses that reflect suprahomeostatic concentrations achieved in lymphopenic hosts, is a potent and selective inducer of the gut-homing integrin α4β7 in human T cells, as documented both ex vivo and in vivo in patients enrolled in a clinical trial of IL-7 treatment. Induction of α4β7 by IL-7 occurs primarily in naive T cells and is associated with functional activation of the integrin, as indicated by increased binding activity for the specific α4β7 ligand, MAdCAM-1. The physiologic relevance of these findings was validated by the preferential homing of IL-7–treated naive human T cells to the intestinal compartment in humanized NOD/SCID/IL-2 receptor-γnull (NSG) mice. We also show that IL-7 triggers a peculiar activation program in naive T cells, characterized by the acquisition of memory-like phenotypic features and proliferation uncoupled from expression of classic T-cell activation markers. These findings provide a mechanism for the transient in vivo depletion of circulating T cells after IL-7 administration and suggest that intestinal homing and memory-like conversion of naive T cells are critical steps in the IL-7–driven immunologic reconstitution of lymphopenic hosts.
Introduction
Interleukin-7 (IL-7) is a nonredundant cytokine that plays an essential role in lymphopoiesis and in the homeostasis of the T-lymphoid compartment in adults.1,2 IL-7 is produced at constitutive levels by stromal cells resident in various organs, as well as by thymic and intestinal epithelial cells.3 Under physiologic conditions, IL-7 supports long-term survival of naive and memory T cells without inducing proliferation, thereby maintaining the regular size of the T-cell pool.2 Under conditions of lymphopenia, the concentration of IL-7 rises to suprahomeostatic levels (as reflected by plasma concentrations greater than 10 pg/mL) that induce proliferation of both naive and memory T cells with the aim of reconstituting the physiologic T-cell pool, a process commonly referred to as lymphopenia-induced proliferation.2 Because of these unique biologic properties and lack of side effects typically associated with other cytokines such as IL-2, IL-7 is currently under clinical evaluation as an immune-reconstitution agent in various forms of immunodeficiencies, including those associated with AIDS and cancer.4 Short-term courses of IL-7 administration in humans and macaques were shown to result in proliferation of both CD4+ and CD8+ T cells, with preferential expansion of naive T cells associated with increased diversification of the T-cell receptor (TCR) repertoire.5-9 Remarkably, injection of IL-7 induces a rapid, albeit transient, reduction in circulating lymphocyte counts compatible with redistribution of these cells to peripheral tissues.6,7 This phenomenon may reflect events that occur naturally when endogenous IL-7 increases to suprahomeostatic levels in response to lymphopenia. Although data obtained in macaques have suggested that lymph nodes, parts of the intestine, and the skin may be homing sites for T cells after IL-7 injection,10 the anatomical sites where homeostatic processes take place and the molecular mechanisms underlying the IL-7–driven homing and proliferation of T cells in peripheral tissues remain largely undefined.
The circulation of T cells from blood to secondary lymphoid organs and other tissues is governed by a complex network of tissue-homing mechanisms, which mainly relies on integrins, chemokine receptors, and their specific ligands.11 In this study, we show that IL-7 selectively induces expression and functional activation of integrin α4β7, the main intestinal lymphocyte homing receptor,11-14 both in vitro and in vivo. This effect occurs predominantly in naive T cells, which indeed showed a marked and selective in vivo homing to the intestinal compartment of humanized mice after treatment with IL-7. The evidence presented in this study provides a mechanism for the rapid reduction of circulating T cells after in vivo IL-7 administration and suggests that α4β7-mediated gut homing of naive T cells may be a fundamental step in the IL-7–driven T-cell reconstitution of lymphopenic hosts.
Methods
Cells and culture conditions
Peripheral blood mononuclear cells (PBMCs) from healthy adult blood donors and patients enrolled in the ACTG5214 clinical trial were isolated from leukapheresis packs using lymphocyte separation medium (LSM; MP Biomedicals). T-cell subpopulations (CD4+, CD8+, naive, and memory) were separated by negative selection using magnetic beads (StemCell Technologies) to > 95% purity, as determined by flow cytometry. Recombinant human (rh) IL-7 (Peprotech) or rhIL-2 (Roche) were added at the doses indicated in each experiment. Details about the culture conditions, multicolor flow cytometry, immunoblotting, antibodies used, and statistical analysis are available in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Tissue homing in humanized NSG mice
NOD/SCID/IL-2 receptor-γnull (NSG) mice (7 to 14 weeks old; The Jackson Laboratory) were reconstituted by intraperitoneal injection of PBMCs (10 × 106/mouse) from 2 healthy donors 30 days before the homing experiment. Mice were housed under specific-pathogen-free conditions and used in accordance with the guidelines of the Institutional Animal Care Committee at the National Institutes of Health (NIH). The protocol was approved by the NIH Animal Care and Use Committee and written informed consent was obtained from all participants according to the Declaration of Helsinki (NCT 00099671). Mice were reconstituted to avoid the potential interference of endogenous murine IL-7, whose levels naturally increase in immunodeficient mice, as well as to create a favorable environment for T-cell tissue homing, which is impaired when peripheral lymphoid tissue is totally absent. Two days before the experiment, purified CD4+ T cells obtained from the same blood donors were treated with IL-7 (25 ng/mL) or left untreated in RPMI supplemented with mouse serum to minimize nonspecific cell activation and loaded with the vital dyes CellTrace Violet or CellTrace CFSE (Invitrogen), respectively, each at 500nM. After 48 hours, the cells were mixed at a 1:1 ratio and transferred (80 × 106 cells/mouse) by intravenous injection into autologously humanized mice. The presence of human T cells in different tissues was evaluated using specific gating on vital dye-labeled cells and further confirmed by testing expression of human CD3 and CD4. Eighteen hours after the transfer, the mice were killed, different organs were collected and disrupted by teasing, and total cell suspensions were made by gently mashing the debris through 70mM nylon mesh filters (BD Bioscience). T cells from the intestinal compartment were isolated using a collagenase-based method.15 The distribution of T-cell populations was determined by cell-surface staining and flow cytometry.
Results
IL-7 induces expression of the gut-homing integrin α4β7 in human T cells
To investigate the molecular mechanisms of IL-7–mediated peripheral homing, we evaluated changes in the expression of a wide panel of integrin subunits in PBMCs derived from healthy adult blood donors treated ex vivo with IL-7 in the absence of any other concomitant stimulation. IL-7, used at 5 ng/mL, induced a marked up-regulation of α4 and β7expression in both CD4+ and CD8+ T cells analyzed by individual gating by multicolor flow cytometry (Figure 1A). Among the other integrins tested, the majority (ie, α1, α2, α3, α5, α6, αE, αL, and β2) were not modified by IL-7 treatment (R.C., L.V. and P.L., unpublished data, 2009), with only β1 showing a moderate up-regulation (Figure 1A). The mean-fold increases for β7and β1 on IL-7 treatment were 171% and 33%, respectively, for CD4+ T cells, and 169% and 17%, respectively, for CD8+ T cells. Of note, the β7+ population expressed low levels of β1, whereas the β1high population was negative for β7 expression. Similar results were obtained by treating with IL-7 purified CD4+ and CD8+ T-cell populations (Figure 1B), thus ruling out an indirect effect mediated by other cell subsets present in PBMC cultures. As controls, we tested the effects of IL-2 and IL-15, 2 other interleukins of the common γ-chain family involved in the development and proliferation of the T-lymphoid compartment, but we did not detect major modifications of either α4 or β7 expression (R.C. and P.L., unpublished data, 2010).
Ex vivo treatment with IL-7 induces integrin α4β7 in human CD4+ and CD8+ T cells. (A) Expression of different α and β integrins as evaluated by flow cytometry in PBMCs derived from healthy blood donors after 72 hours of treatment with IL-7 at 5 ng/mL; control cultures (C) were not treated. Data are representative of 3 independent experiments performed with analogous results. The shaded histograms denote staining with the indicated antibody; the empty profiles denote the background fluorescent signal obtained with an irrelevant, isotype-matched antibody. CD4+ and CD8+ T cells were separately analyzed by differential gating using multicolor flow cytometry. The numbers in each plot indicate the mean fluorescence intensity (MFI) of the stained population. (B) Expression of α4 and β7 in IL-7–treated cells (shaded histogram) or untreated (empty profiles) cells as evaluated by flow cytometry using either subunit-specific antibodies or an antibody that recognizes the heterodimeric α4β7 complex (clone Act1). CD4+ and CD8+ T cells were purified from the peripheral blood of healthy blood donors and cultured for 72 hours in the presence or absence of IL-7 at 5 ng/mL. Data are representative of 3 independent experiments performed with similar results. (C) Mean levels of expression (± standard error of the mean) of the α4β7 heterodimer in purified CD4+ T cells from 25 healthy blood donors treated for 72 hours with IL-7 (5 ng/mL) or left untreated (control). Statistical analysis was performed using a paired 2-tailed t test. (D) Effect of IL-7 on α4β7 expression in NK and B cells. PBMCs derived from a healthy blood donor were treated with IL-7 at 5 ng/mL or left untreated for 72 hours, and the expression of α4β7 was measured by flow cytometry. Data for gated NK cells (CD56+) and B cells (CD19+) are shown. The shaded histograms denote the staining of IL-7–treated cells; the empty profiles denote the staining of control, untreated cells. Data are representative of at least 3 independent experiments performed with similar results. (E) Effect of IL-7 and RA on the expression of integrin α4β7 in purified CD4+ T cells. The cells were treated with IL-7 (5 ng/mL) or RA (10μM) or a combination of IL-7 and RA for 72 hours (shaded histograms) or left untreated (empty profiles) in the presence or absence of anti-CD3 + anti-CD28 antibodies. Data are representative of at least 3 independent experiments performed with similar results. (F) Expression of the IL-7 receptor (CD127) in purified CD4+ T cells treated with IL-7 or RA (shaded histograms) or left untreated (empty profiles) for 72 hours in the presence or absence of anti-CD3 + anti-CD28 antibodies. Data are representative of 3 independent experiments performed with similar results. (G) Expression of chemokine receptors CCR7, CCR9, and CXCR4 in PBMCs from healthy blood donors after 72 hours of treatment with IL-7 (5 ng/mL); control cultures (C) were not treated. Data are representative of 3 independent experiments performed with analogous results. The shaded histograms denote staining with the indicated antibody; the empty profiles denote the background fluorescent signal obtained with an irrelevant, isotype-matched antibody. The numbers in each plot indicate the MFI of the stained population.
Ex vivo treatment with IL-7 induces integrin α4β7 in human CD4+ and CD8+ T cells. (A) Expression of different α and β integrins as evaluated by flow cytometry in PBMCs derived from healthy blood donors after 72 hours of treatment with IL-7 at 5 ng/mL; control cultures (C) were not treated. Data are representative of 3 independent experiments performed with analogous results. The shaded histograms denote staining with the indicated antibody; the empty profiles denote the background fluorescent signal obtained with an irrelevant, isotype-matched antibody. CD4+ and CD8+ T cells were separately analyzed by differential gating using multicolor flow cytometry. The numbers in each plot indicate the mean fluorescence intensity (MFI) of the stained population. (B) Expression of α4 and β7 in IL-7–treated cells (shaded histogram) or untreated (empty profiles) cells as evaluated by flow cytometry using either subunit-specific antibodies or an antibody that recognizes the heterodimeric α4β7 complex (clone Act1). CD4+ and CD8+ T cells were purified from the peripheral blood of healthy blood donors and cultured for 72 hours in the presence or absence of IL-7 at 5 ng/mL. Data are representative of 3 independent experiments performed with similar results. (C) Mean levels of expression (± standard error of the mean) of the α4β7 heterodimer in purified CD4+ T cells from 25 healthy blood donors treated for 72 hours with IL-7 (5 ng/mL) or left untreated (control). Statistical analysis was performed using a paired 2-tailed t test. (D) Effect of IL-7 on α4β7 expression in NK and B cells. PBMCs derived from a healthy blood donor were treated with IL-7 at 5 ng/mL or left untreated for 72 hours, and the expression of α4β7 was measured by flow cytometry. Data for gated NK cells (CD56+) and B cells (CD19+) are shown. The shaded histograms denote the staining of IL-7–treated cells; the empty profiles denote the staining of control, untreated cells. Data are representative of at least 3 independent experiments performed with similar results. (E) Effect of IL-7 and RA on the expression of integrin α4β7 in purified CD4+ T cells. The cells were treated with IL-7 (5 ng/mL) or RA (10μM) or a combination of IL-7 and RA for 72 hours (shaded histograms) or left untreated (empty profiles) in the presence or absence of anti-CD3 + anti-CD28 antibodies. Data are representative of at least 3 independent experiments performed with similar results. (F) Expression of the IL-7 receptor (CD127) in purified CD4+ T cells treated with IL-7 or RA (shaded histograms) or left untreated (empty profiles) for 72 hours in the presence or absence of anti-CD3 + anti-CD28 antibodies. Data are representative of 3 independent experiments performed with similar results. (G) Expression of chemokine receptors CCR7, CCR9, and CXCR4 in PBMCs from healthy blood donors after 72 hours of treatment with IL-7 (5 ng/mL); control cultures (C) were not treated. Data are representative of 3 independent experiments performed with analogous results. The shaded histograms denote staining with the indicated antibody; the empty profiles denote the background fluorescent signal obtained with an irrelevant, isotype-matched antibody. The numbers in each plot indicate the MFI of the stained population.
Integrins can only be expressed on the cellular surface as heterodimers formed by an α and a β subunit. α4 can couple with either β7 or β1, whereas β7 can couple with either α4 or αE.12 No expression of αE was detected on the surface of primary T cells regardless of IL-7 treatment, whereas β1 was up-regulated by IL-7, but not nearly as dramatically as was β7 (Figure 1A), suggesting that the bulk of α4 expressed by IL-7–treated cells was heterodimerized with β7. Indeed, using a monoclonal antibody (mAb) specific for the α4β7 heterodimeric complex (clone Act-1), we observed that IL-7 induced up-regulation of the α4β7heterodimer, which paralleled the up-regulation of α4 and β7 tested individually (Figure 1B). The effect of IL-7 was highly reproducible in purified CD4+ T cells from 25 healthy blood donors tested, resulting in a highly significant increase (P < .0001) in expression of the α4β7 heterodimer (Figure 1C). The effect was restricted to T cells as treatment with IL-7 did not alter the expression of α4β7 on either NK cells or B cells (Figure 1D). Because retinoic acid (RA) is believed to play a critical role in the in vivo induction of α4β7, priming T cells for intestinal homing,16,17 we tested the ability of all-trans RA to modulate α4β7 expression in purified CD4+ and CD8+ T cells under the same experimental conditions used for IL-7. In the absence of T-cell receptor (TCR)–mediated stimulation, RA at 10μM did not affect the expression of α4β7, and the addition of RA to IL-7 did not augment the effect of IL-7; conversely, as reported,17 RA induced significant α4β7 up-regulation when used in combination with anti-CD3/CD28 antibodies (Figure 1E). Remarkably, in cells stimulated with anti-CD3/CD28 antibodies, IL-7 did not induce α4β7 expression (Figure 1E). This lack of IL-7 responsiveness was associated with a dramatic downmodulation of the IL-7 receptor (CD127) in TCR-activated T cells (Figure 1F), as reported.18
In addition to integrins, chemokine receptors also play a role in the homing of lymphocytes to secondary lymphoid organs. Thus, we evaluated the effects of IL-7 on the expression of a panel of chemokine receptors. As previously documented,19,20 IL-7 induced a marked increase in the expression of CXCR4, a homeostatic chemokine receptor involved in peripheral T-cell homing but lacking organ specificity,11,21 whereas the expression of CCR9, a chemokine receptor involved in intestinal homing,13 was only slightly up-regulated (Figure 1G). None of the other chemokine receptors analyzed (ie, CCR2, CCR3, CCR4, CCR5, CCR6, CXCR3) was modified by IL-7 treatment (R.C., P.L., unpublished data), with the exception of CCR7, the main chemokine receptor involved in lymph node homing,11,21 which was moderately down-regulated (Figure 1G).
Induction of α4β7 by IL-7 occurs primarily in naive T cells
Because naive, central memory (CM), and effector memory (EM) T cells differ in their responses to IL-7,2 we investigated the ability of IL-7 to modify the expression of α4β7 on individual T-cell subpopulations. Before IL-7 stimulation, naive T cells typically express an intermediate level of the α4β7 heterodimer (α4β7int), whereas the majority of CM and EM T cells are negative (α4β7neg) with a variable proportion (between 10% and 30%) expressing high levels of α4β7 (α4β7hi; Figure 2). After treatment with IL-7, α4β7 was prominently up-regulated in naive CD4+ and CD8+ T cells, with a marked shift of the entire cell population from α4β7int to α4β7hi; in contrast, among CM and EM CD4+ and CD8+ T cells, the larger population that was α4β7neg at baseline was unaffected by IL-7, whereas the small population that was already α4β7hi showed some degree of up-regulation (Figure 2). Thus, IL-7 confers on naive T cells an α4β7hi phenotype, which is associated with homing to the intestinal compartment.12,13
Induction of α4β7 by IL-7 occurs primarily in naive T cells. Purified CD4+ and CD8+ T cells were cultured in the presence or absence of IL-7 at 5 ng/mL. After 24 hours of culture, the expression of α4β7 was evaluated in the naive (CD45RA+CD45RO−CCR7+CD62L+), central memory (CM; CD45RA−CD45RO+CCR7+CD62L+) and effector memory (EM; CD45RA−CD45RO+CCR7−CD62L±) cell subsets by flow cytometry. The shaded histograms denote the staining of IL-7–treated cells; the empty profiles denote the staining of control, untreated cells. Data are representative of 3 independent experiments performed with similar results.
Induction of α4β7 by IL-7 occurs primarily in naive T cells. Purified CD4+ and CD8+ T cells were cultured in the presence or absence of IL-7 at 5 ng/mL. After 24 hours of culture, the expression of α4β7 was evaluated in the naive (CD45RA+CD45RO−CCR7+CD62L+), central memory (CM; CD45RA−CD45RO+CCR7+CD62L+) and effector memory (EM; CD45RA−CD45RO+CCR7−CD62L±) cell subsets by flow cytometry. The shaded histograms denote the staining of IL-7–treated cells; the empty profiles denote the staining of control, untreated cells. Data are representative of 3 independent experiments performed with similar results.
Induction of α4β7 by IL-7 is dose and time dependent
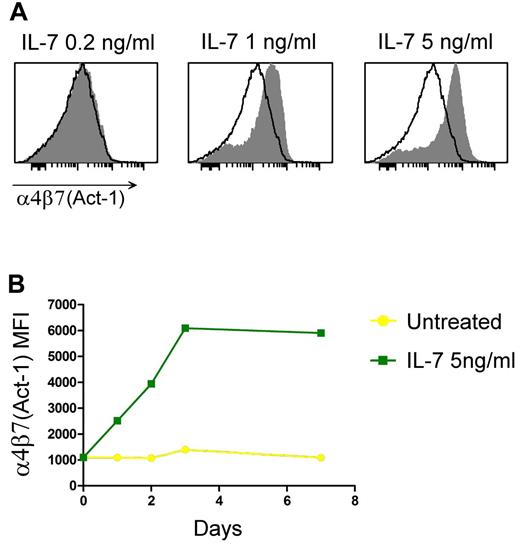
Next, we investigated whether α4β7 up-regulation by IL-7 is dependent on the cytokine concentration using purified naive CD4+ T cells, which were the most responsive subset to IL-7 stimulation. A distinct threshold effect was observed, with IL-7 concentrations equal to or greater than 1 ng/mL inducing up-regulation of α4β7, whereas doses below 1 ng/mL failed to alter the level of integrin expression (Figure 3A). Only a single healthy subject of more than 25 tested displayed a hyper-responsive phenotype, with significant up-regulation of α4β7 with IL-7 used at 0.2 ng/mL; the maximal effect was typically observed at 5 ng/mL, whereas higher concentrations did not further enhance the expression of the integrin (R.C. and P.L., unpublished data, 2010).
IL-7–induced α4β7 expression is dose and time dependent. (A) Dose-response effects of IL-7 on α4β7 expression in purified naive CD4+ T lymphocytes after 72 hours of culture. The shaded histograms denote the staining of IL-7–treated cells; the empty profiles denote the staining of control, untreated cells. Data are representative of 3 independent experiments performed with similar results. (B) Expression of α4β7 as evaluated by flow cytometry over a period of 7 days in purified naive CD4+ T cells that were either untreated or treated with IL-7 at 5 ng/mL. The values of MFI are shown. Data are representative of 3 independent experiments performed with similar results.
IL-7–induced α4β7 expression is dose and time dependent. (A) Dose-response effects of IL-7 on α4β7 expression in purified naive CD4+ T lymphocytes after 72 hours of culture. The shaded histograms denote the staining of IL-7–treated cells; the empty profiles denote the staining of control, untreated cells. Data are representative of 3 independent experiments performed with similar results. (B) Expression of α4β7 as evaluated by flow cytometry over a period of 7 days in purified naive CD4+ T cells that were either untreated or treated with IL-7 at 5 ng/mL. The values of MFI are shown. Data are representative of 3 independent experiments performed with similar results.
Time-course analysis demonstrated that up-regulation of α4β7 by IL-7 occurs with fast kinetics, becoming detectable within 24 hours of treatment and reaching a peak between 48 and 72 hours depending on the donor tested (Figure 3B). In contrast, no increase was seen within the first 6 hours of treatment (R.C. and P.L., unpublished data, 2010). This kinetic correlates well with the rapid decrease in the number of circulating T cells documented in vivo after a single subcutaneous administration of IL-7.7,10 Prolonged follow-up of the cultures demonstrated that the effects of IL-7 on α4β7 are long-lasting because the integrin remained up-regulated for more than 7 days after a single initial stimulation (Figure 3B) even when IL-7 was removed after the first 48 hours of treatment (R.C. and P.L., unpublished data, 2010).
Differential α4β7 induction by IL-7 in memory and naive T cells is not associated with differential expression of the IL-7 receptor
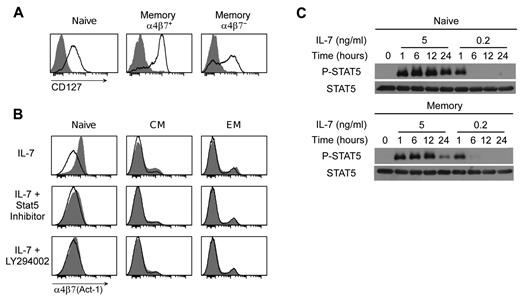
To determine whether the differential ability of IL-7 to induce α4β7 on various T-cell subsets was correlated with expression of the IL-7 receptor (IL-7R), we analyzed the expression of CD127 in freshly isolated, unstimulated CD4+ T cells. CD127 was highly expressed on the majority of α4β7hi memory CD4+ T cells and at an intermediate level on naive CD4+ T cells, whereas α4β7neg memory T cells included both CD127+ and CD127− cells with the majority expressing intermediate-high levels of the receptor (Figure 4A empty profiles). Within 24 hours of IL-7 stimulation, CD127 was completely down-regulated in naive and memory T cells alike (Figure 4A shaded histograms), indicating that despite the lack of α4β7 induction by IL-7 most memory T cells express a functional IL-7R on their surface.
IL-7–induced up-regulation α4β7 is unrelated to expression of the IL-7 receptor and depends on activation of both the JAK-STAT and PI3K signaling pathways. (A) Expression of the α-chain of the IL-7 receptor (CD127) in naive, memory α4β7+ and memory α4β7− CD4+ T cells as evaluated by flow cytometry in purified CD4+ T cells cultured for 24 hours in the presence or absence of IL-7 at 5 ng/mL. The shaded histograms denote the staining of IL-7–treated cells; the empty profiles denote the staining of untreated cells. (B) Inhibition of IL-7–mediated α4β7 induction in naive, CM and EM CD4+ T cells by specific Stat5 or PI3K inhibitors. Both inhibitors were used at 50μM; the cells were analyzed by flow cytometry using mAb Act-1 after 24 hours of culture in the presence of IL-7. Because both inhibitors were dissolved in DMSO, cells cultured in the absence of inhibitors were treated with equivalent amounts of DMSO (1:1000 vol/vol). The different CD4+ T-cell subsets were separately analyzed based on the expression of CD45RA, CD45RO, CD62L, and CCR7. The shaded histograms denote the staining of cytokine-treated cells; the empty profiles denote the staining of control, untreated cells. (C) Phosphorylation of Stat5 (P-STAT) in purified naive and memory CD4+ T lymphocytes exposed to different doses of IL-7 as tested by Western blot at various time points over a period of 24 hours. Time 0 indicates the baseline levels of expression before IL-7 treatment. The total amount of Stat protein (STAT) was measured in parallel in the same samples. Data are representative of at least 3 independent experiments performed with similar results.
IL-7–induced up-regulation α4β7 is unrelated to expression of the IL-7 receptor and depends on activation of both the JAK-STAT and PI3K signaling pathways. (A) Expression of the α-chain of the IL-7 receptor (CD127) in naive, memory α4β7+ and memory α4β7− CD4+ T cells as evaluated by flow cytometry in purified CD4+ T cells cultured for 24 hours in the presence or absence of IL-7 at 5 ng/mL. The shaded histograms denote the staining of IL-7–treated cells; the empty profiles denote the staining of untreated cells. (B) Inhibition of IL-7–mediated α4β7 induction in naive, CM and EM CD4+ T cells by specific Stat5 or PI3K inhibitors. Both inhibitors were used at 50μM; the cells were analyzed by flow cytometry using mAb Act-1 after 24 hours of culture in the presence of IL-7. Because both inhibitors were dissolved in DMSO, cells cultured in the absence of inhibitors were treated with equivalent amounts of DMSO (1:1000 vol/vol). The different CD4+ T-cell subsets were separately analyzed based on the expression of CD45RA, CD45RO, CD62L, and CCR7. The shaded histograms denote the staining of cytokine-treated cells; the empty profiles denote the staining of control, untreated cells. (C) Phosphorylation of Stat5 (P-STAT) in purified naive and memory CD4+ T lymphocytes exposed to different doses of IL-7 as tested by Western blot at various time points over a period of 24 hours. Time 0 indicates the baseline levels of expression before IL-7 treatment. The total amount of Stat protein (STAT) was measured in parallel in the same samples. Data are representative of at least 3 independent experiments performed with similar results.
Both the JAK-STAT and PI3K signaling pathways are required for α4β7 induction by IL-7
Binding of IL-7 to the IL-7R triggers activation of the JAK-STAT and the PI3K signaling pathways.22 To identify the intracellular signaling pathway(s) implicated in α4β7 up-regulation by IL-7, purified CD4+ T cells were treated with IL-7 for 24 hours in the presence of specific JAK-STAT or PI3K inhibitors. The specificity of the inhibitors was proven by their dose-dependent activity on the respective signaling pathway in the absence of cellular toxicity (R.C. and P.L., unpublished data, 2011). Both inhibitors effectively prevented α4β7 up-regulation by IL-7 (Figure 4B), demonstrating that this effect depends on the activation of both signaling pathways.
Because we observed that induction of α4β7 by IL-7 is a dose-dependent phenomenon with a defined threshold, we studied the effects of different IL-7 concentrations on Stat5 activation in purified naive and memory CD4+ T cells. At a low concentration (0.2 ng/mL), which does not induce α4β7, IL-7 elicited short-lived phosphorylation of tyrosine 694 in Stat5, lasting for less than 6 hours, whereas at a higher concentration (5 ng/mL), which induces α4β7, there was sustained Stat5 phosphorylation that was still detectable at 24 hours after IL-7 treatment, with similar kinetics in memory and naive T cells (Figure 4C). These data demonstrated a correlation between the IL-7 doses that induce α4β7 up-regulation and those that induce sustained Stat5 activation.
IL-7 induces functional activation of α4β7
The integrin function of α4β7 is critically dependent not only on a high level of expression but also on the adoption of an active conformation.23 Although the majority of circulating T cells express α4β7, most of the heterodimers present on the cellular surface are in an inactive conformation.23 To evaluate whether the α4β7hi phenotype induced by IL-7 correlated with functional activation of the integrin, we tested the ability of soluble recombinant human mucosal addressin cell adhesion molecule-1 (MAdCAM-1), the specific ligand of α4β7, to bind purified CD4+ T cells treated with IL-7 for 24 hours; RA, either alone or in combination with IL-7, was tested in parallel as a control. Because MAdCAM-1 binding is critically affected by the concentration of divalent cations, binding was assayed at physiologic concentrations of Mg++, which permit to evaluate the effective activation state of the integrin. Among untreated cells, the small proportion of memory CD4+ T cells that display an α4β7hi phenotype was the only subset to show efficient MAdCAM-1 binding, whereas the majority of naive CD4+ T cells were unable to bind MAdCAM-1 despite their homogeneous α4β7int expression (Figure 5A empty profiles). MAdCAM binding was dramatically increased in naive CD4+ T cells treated with IL-7, whereas among memory CD4+ T cells the increase was restricted to the α4β7hi subpopulation (Figure 5A shaded histograms); binding was abrogated by a neutralizing mAb to α4β7 (Figure 5A red hatched histograms). Treatment with RA did not modify the levels of MAdCAM-1 binding nor augment the effect of IL-7 when used in combination (Figure 5A). Thus, IL-7 induces not only up-regulation but also functional activation of the α4β7 heterodimer, which is required for homing to the intestinal tissue.
IL-7 induces functional activation of α4β7 and migration of naive human T cells to the intestinal compartment in humanized NSG mice. (A) Binding of the specific α4β7 ligand MAdCAM-1 to purified memory and naive CD4+ T cells treated for 24 hours with the indicated cytokines or left untreated. The shaded histograms denote the staining of cytokine-treated cells; the empty profiles denote the staining of control, untreated cells; the red hatched histograms denote staining in the presence of the neutralizing anti-α4 mAb HP2/1. Data are representative of 3 independent experiments performed with similar results. (B) Expression of integrin α4β7 in T cells derived from the blood, spleen, inguinal lymph nodes (iLN) and mesenteric lymph nodes (mLN) of wild-type C57BL/6 mice injected subcutaneously with murine IL-7 (1 or 5 μg/mouse) or treated with placebo (control). Mean values (± SEM) from 2 mice tested for each condition are shown. (C) Tissue homing of IL-7–treated naive and memory human T cells injected into humanized NSG mice. Human CD4+ T cells purified from healthy blood donors and treated ex vivo with IL-7 for 48 hours were loaded with a vital dye, mixed at 1:1 ratio with untreated autologous cells loaded with a different vital dye, and then injected into the tail vein of 6 NSG mice previously repopulated with autologous PBMCs. After 18 hours, the mice were killed, and the relative proportion of IL-treated versus untreated cells was quantitated in blood and peripheral tissues. Naive and memory T cells were separately analyzed by differential gating based on the expression of human CD45RA and CD45RO. The data indicate the mean percent increase (± SEM) in the number of recovered IL-7–treated cells over untreated cells for the indicated T-cell subset from the tissues of 6 mice. Statistical analysis was performed using a Wilcoxon matched-pairs test. The numbers in parentheses denote the proportion of total naive and memory human T cells recovered in the indicated tissue.
IL-7 induces functional activation of α4β7 and migration of naive human T cells to the intestinal compartment in humanized NSG mice. (A) Binding of the specific α4β7 ligand MAdCAM-1 to purified memory and naive CD4+ T cells treated for 24 hours with the indicated cytokines or left untreated. The shaded histograms denote the staining of cytokine-treated cells; the empty profiles denote the staining of control, untreated cells; the red hatched histograms denote staining in the presence of the neutralizing anti-α4 mAb HP2/1. Data are representative of 3 independent experiments performed with similar results. (B) Expression of integrin α4β7 in T cells derived from the blood, spleen, inguinal lymph nodes (iLN) and mesenteric lymph nodes (mLN) of wild-type C57BL/6 mice injected subcutaneously with murine IL-7 (1 or 5 μg/mouse) or treated with placebo (control). Mean values (± SEM) from 2 mice tested for each condition are shown. (C) Tissue homing of IL-7–treated naive and memory human T cells injected into humanized NSG mice. Human CD4+ T cells purified from healthy blood donors and treated ex vivo with IL-7 for 48 hours were loaded with a vital dye, mixed at 1:1 ratio with untreated autologous cells loaded with a different vital dye, and then injected into the tail vein of 6 NSG mice previously repopulated with autologous PBMCs. After 18 hours, the mice were killed, and the relative proportion of IL-treated versus untreated cells was quantitated in blood and peripheral tissues. Naive and memory T cells were separately analyzed by differential gating based on the expression of human CD45RA and CD45RO. The data indicate the mean percent increase (± SEM) in the number of recovered IL-7–treated cells over untreated cells for the indicated T-cell subset from the tissues of 6 mice. Statistical analysis was performed using a Wilcoxon matched-pairs test. The numbers in parentheses denote the proportion of total naive and memory human T cells recovered in the indicated tissue.
IL-7 induces intestinal homing of naive human T cells in reconstituted NSG mice
The observed functional activation of α4β7 suggested that IL-7 may confer on T cells the ability to migrate to the intestinal mucosa. To validate this hypothesis, we initially considered performing in vivo T-cell homing studies in a murine model. Surprisingly, however, we found that α4β7 expression in primary mouse T cells was not affected by ex vivo treatment with recombinant murine or human IL-7; likewise, in vivo injection of murine IL-7 into C57BL/6 mice failed to induce up-regulation of α4β7 expression in T cells from blood, spleen, and lymph nodes (Figure 5B). To overcome these limitations, we opted for a chimeric model in which human CD4+ T cells, which efficiently up-regulate α4β7 in response to IL-7, were adoptively transferred into NSG mice reconstituted with autologous human immune cells. Of note, we showed that murine MAdCAM-1 binds human α4β7 with the same efficiency as human MAdCAM-1 (R.C. and P.L., unpublished data, 2011). Four weeks before the in vivo transfer, the mice were reconstituted by intraperitoneal injection of total PBMC derived from 2 healthy volunteers. Two days before the transfer, the same 2 blood donors used for reconstitution were recalled and subjected to leukapheresis, and their CD4+ T cells were isolated and cultured ex vivo in the presence or absence of IL-7 for 48 hours. IL-7–treated and untreated cells were then labeled with different vital dyes, mixed at 1:1 ratio, and injected into the tail vein of autologous reconstituted mice. Quality controls confirmed efficient vital dye uptake and α4β7 induction by IL-7 (supplemental Figure 1). Eighteen hours after injection, the mice were killed, and the tissue distribution of IL-7–treated versus untreated human T cells was evaluated. In agreement with the preferential induction and activation of α4β7 in naive T cells by IL-7, we found that a significantly higher proportion of IL-7–treated human naive T cells migrated to the intestinal mucosa, compared with untreated naive T cells, whereas memory T cells accounted for only a minor fraction of total human T cells migrated to the intestine with equal proportions of IL-7–treated and untreated cells (Figure 5C). Analysis of peripheral blood documented a decrease in both naive and memory IL-7–treated T cells, indicating that IL-7 promoted the extravasation of all T cells, although only the naive subset was specifically targeted to the intestine; analysis of the spleen and bone marrow showed a general reduction of IL-7–treated memory T cells, compared with their untreated counterparts, whereas IL-7–treated naive T cells were less represented in the spleen but not in the blood and bone marrow (Figure 5C). Although reconstituted mice showed the formation of initial lymph node-like structures, the lymphocyte recovery from such structures was too low to permit a reliable analysis of human T-cell homing (R.C. and P.L., unpublished data, 2011). Altogether, these results demonstrated that IL-7 treatment specifically induces intestinal homing of naive T cells, providing a physiologic validation for the selective induction and activation of α4β7 on this T-cell subset by IL-7.
IL-7 induces homeostatic T-cell proliferation uncoupled from T-cell activation
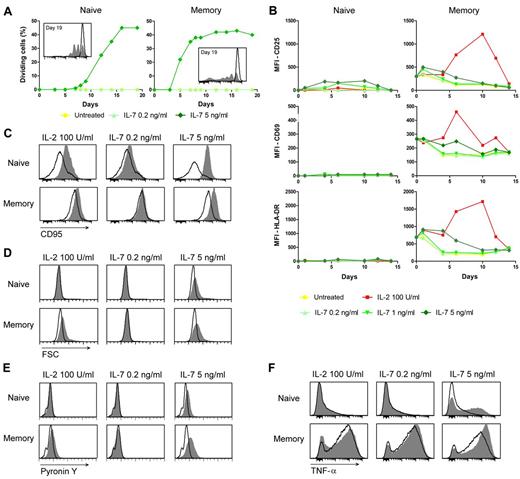
Because IL-7 plays a critical role in homeostatic T-cell proliferation in response to lymphopenia, and in vivo administration of IL-7 induces transient T-cell proliferation,2,5,7,8 we evaluated the proliferative effects of IL-7 on naive and memory T cells, as well as the correlation between proliferation and α4β7 induction. Treatment with IL-7 at 5 ng/mL, a dose that induces α4β7, in the absence of concomitant TCR-mediated stimulation triggered proliferation of both naive and memory T cells, whereas at 0.2 ng/mL, a dose that does not alter α4β7 expression, no cell division was detected in either cell subset over a period of 19 days in culture (Figure 6A). Naive T cells showed delayed kinetics of proliferation, compared with memory T cells (starting on day 8 after treatment vs day 5 in memory cells), but they reached similar proportions of dividing cells (∼ 40%) after 2 weeks of ex vivo stimulation; of note, both α4β7hi and α4β7neg memory T cells proliferated, further emphasizing the lack of correlation between α4β7 induction by IL-7 and expression of a functionally competent IL-7R. Thus, IL-7 can elicit proliferation of both naive and memory T cells in the absence of any concomitant stimulation at the same doses that also induce α4β7.
IL-7 induces proliferation of both naive and memory T cells uncoupled from T-cell activation, and memory-like phenotypic features in naive T cells. (A) Proliferation of naive and memory T cells treated with IL-7. Purified naive and memory CD4+ T cells were stained with CFSE and cultured in the presence or absence of low (0.2 ng/mL) or high (5 ng/mL) concentrations of IL-7. The lines represent the proportion of proliferating cells as evaluated by CFSE dilution over a period of 19 days in culture. The histograms in the insert show CSFE staining at day 19 in untreated (empty profile) and IL-7–treated (5 ng/mL; shaded histogram) CD4+ T lymphocytes. Data are representative of 3 independent experiments performed with similar results. (B) Expression of T-cell activation markers as evaluated by flow cytometry in purified naive and memory CD4+ T cells untreated or treated with the indicated cytokines over a period of 14 days. The values of MFI are shown for each marker at the indicated time points. Data are representative of 3 independent experiments performed with similar results. (C-F) Expression of memory-associated markers in naive and memory CD4+ T cells treated with IL-7. Purified naive and memory CD4+ T cells were left untreated or treated with the indicated doses of IL-7 and analyzed by flow cytometry after 3 days of culture for CD95 expression (C), cellular size (forward scatter, FSC; D), RNA content (pyronin Y; E), and TNF-α secretion (F). The ability to produce TNF-α was evaluated by intracellular staining followed by flow cytometry analysis on cells activated for an additional 6 hours with PMA and ionomycin. The shaded histograms denote the staining of IL-7–treated cells; the empty profiles denote the staining of control, untreated cells. Data are representative of 3 independent experiments performed with similar results.
IL-7 induces proliferation of both naive and memory T cells uncoupled from T-cell activation, and memory-like phenotypic features in naive T cells. (A) Proliferation of naive and memory T cells treated with IL-7. Purified naive and memory CD4+ T cells were stained with CFSE and cultured in the presence or absence of low (0.2 ng/mL) or high (5 ng/mL) concentrations of IL-7. The lines represent the proportion of proliferating cells as evaluated by CFSE dilution over a period of 19 days in culture. The histograms in the insert show CSFE staining at day 19 in untreated (empty profile) and IL-7–treated (5 ng/mL; shaded histogram) CD4+ T lymphocytes. Data are representative of 3 independent experiments performed with similar results. (B) Expression of T-cell activation markers as evaluated by flow cytometry in purified naive and memory CD4+ T cells untreated or treated with the indicated cytokines over a period of 14 days. The values of MFI are shown for each marker at the indicated time points. Data are representative of 3 independent experiments performed with similar results. (C-F) Expression of memory-associated markers in naive and memory CD4+ T cells treated with IL-7. Purified naive and memory CD4+ T cells were left untreated or treated with the indicated doses of IL-7 and analyzed by flow cytometry after 3 days of culture for CD95 expression (C), cellular size (forward scatter, FSC; D), RNA content (pyronin Y; E), and TNF-α secretion (F). The ability to produce TNF-α was evaluated by intracellular staining followed by flow cytometry analysis on cells activated for an additional 6 hours with PMA and ionomycin. The shaded histograms denote the staining of IL-7–treated cells; the empty profiles denote the staining of control, untreated cells. Data are representative of 3 independent experiments performed with similar results.
To determine whether IL-7–induced proliferation and α4β7 up-regulation are part of a generalized program of cell activation, we evaluated the expression of T-cell activation markers in T cells treated with IL-7 at both proliferative and nonproliferative concentrations. IL-2 was tested in parallel as a positive control. No expression of 3 activation markers, CD25, CD69, and HLA-DR, was detected in freshly isolated, unstimulated naive CD4+ T cells, and none of these markers was significantly induced at any time after treatment with IL-7 with the exception of modest up-regulation of CD25 with IL-7 at 1 and 5 ng/mL; memory T cells showed higher baseline expression levels of these activation markers, but IL-7 did not induce significant up-regulations (Figure 6B). In contrast, IL-2 elicited a strong and long-lasting up-regulation of all the activation markers in memory T cells, although it had no effects in naive T cells (Figure 6B). These data showed that T-cell proliferation induced by IL-7 is uncoupled from the expression of markers typically associated with TCR-mediated T-cell activation.
IL-7 induces memory-like phenotypic features in naive T cells
Because proliferation of naive T cells on TCR-mediated stimulation is typically associated with a permanent transition to a memory phenotype24 and the acquisition of a memory-like phenotype by naive T cells was previously observed in IL-7–treated macaques,8 we evaluated a panel of memory-associated phenotypic and functional markers in naive T cells treated with different doses of IL-7.25 Within 48 hours of exposure to IL-7 at a proliferative concentration (5 ng/mL), naive CD4+ T cells acquired phenotypic features typical of memory T cells, such as a dramatic up-regulation of CD95 expression (Figure 6C) and increases in both cell size (Figure 6D) and total RNA content (Figure 6E), whereas no effects were seen when IL-7 was used at the nonproliferative concentration of 0.2 ng/mL. This notwithstanding, naive T cells did not acquire, as previously reported,26 a bona fide memory phenotype, as there were no substantial changes in the expression of defining naive/memory markers, such as CD45RA, CD45RO, CD62L, and CCR7 (R.C. and P.L., unpublished data, 2010). In memory CD4+ T cells, IL-7 at 5 ng/mL caused a slight up-regulation of CD95, which is constitutively expressed at high levels in this subset, as well as increases in both cell size and total RNA content (Figure 6C-E). Next, we measured the production of memory-associated cytokines. Strikingly, after treatment with IL-7 at 5 ng/mL, naive CD4+ T cells acquired the ability to produce TNF-α on stimulation with PMA and ionomycin, whereas no cytokine production was detected when the cells were treated with IL-7 at 0.2 ng/mL (Figure 6F). IL-7 at 5 ng/mL, but not at 0.2 ng/mL, also caused a further increase in TNF-α production in memory T cells. Thus, at proliferative concentrations, IL-7 caused naive T cells to acquire a memory-like phenotype despite maintaining a distinctive naive T-cell identity. These results are in line with observations in lymphopenic mice, in which naive T cells transiently acquire a memory-like phenotype while undergoing homeostatic proliferation.27,28
α4β7 is up-regulated in vivo in patients receiving IL-7 treatment
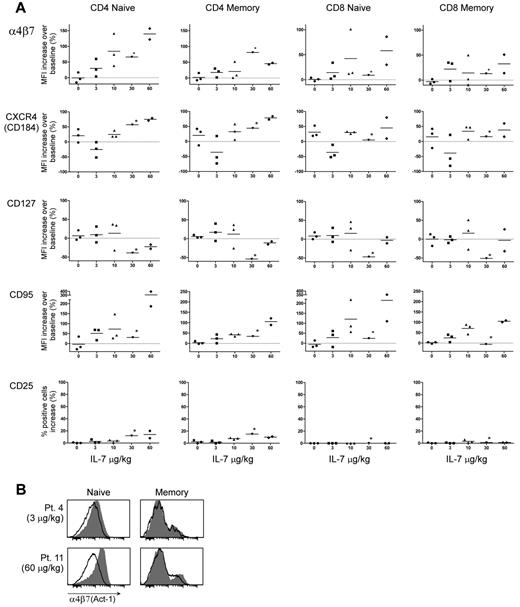
To further investigate the in vivo relevance of our findings, we studied peripheral blood samples obtained from asymptomatic HIV-1–infected patients under antiretroviral therapy enrolled in an unrelated clinical trial of IL-7 treatment, ACTG5214, in which IL-7 administration was shown to induce a rapid reduction in circulating lymphocyte counts.7 Changes in expression of multiple phenotypic markers were evaluated in paired peripheral blood T-cell samples obtained from 12 subjects before and after treatment: 9 of them received IL-7 at different doses (3, 10, 30, or 60 μg/kg), whereas 3 received placebo. Most of the available posttreatment samples were collected 4 days after treatment, although a few were collected on day 1, 7, or 14 after treatment, which allowed us to observe the kinetics of IL-7 effects over time (supplemental Table 1). In agreement with our ex vivo data, IL-7–treated subjects showed a dose-dependent up-regulation of α4β7 expression in both CD4+ and CD8+ T cells, which was more marked in the naive than in the memory subset (Figure 7A). Up-regulation of α4β7 was seen in the entire naive T-cell population, whereas in memory T cells the increase was restricted to a small subpopulation (Figure 7B). The effects of IL-7 were sustained, as shown by persistent up-regulation of α4β7 expression in samples collected on days 7 and 14 after treatment in 2 subjects treated with 60 μg/kg of IL-7. Among the other surface molecules tested, CXCR4 was up-regulated, although the effect was less consistent than that observed ex vivo; CD127 was down-regulated only in 1 subject (treated with 30 μg/kg of IL-7) whose posttreatment sample was collected on day 1, indicating that the IL-7R is rapidly re-expressed after single-dose IL-7 treatment; up-regulation of CD95 and CD25 was only seen in CD4+ T cells and only at the 2 highest IL-7 doses (Figure 7A); in contrast, neither CCR9 nor HLA-DR and CD69 were up-regulated by IL-7 treatment (R.C. and P.L., unpublished data, 2010). Overall, the data obtained from individuals treated with IL-7 in vivo were largely in agreement with our ex vivo findings, confirming that IL-7 treatment alone is sufficient to induce a gut homing and memory-like phenotype in naive T lymphocytes.
In vivo treatment with IL-7 induces α4β7 in humans. (A) Expression of α4β7, CXCR4, CD127, CD95, and CD25 as analyzed by flow cytometry in circulating T cells derived from HIV-1–infected individuals under antiretroviral therapy before and after in vivo injection of the indicated doses of IL-7 or placebo. Naive and memory CD4+ and CD8+ T-cell populations were separately analyzed by multicolor staining and individual gating. For all markers except CD25, each dot represents the percentage increase in expression (MFI) from baseline after the indicated treatment for each of the subjects studied; for CD25 (for which MFI values could be misleading because of a marked shift in fluorescence intensity restricted to a minor subpopulation of cells), the percentage of positive cells is presented. Posttreatment samples were obtained on days 4, 7, or 14 after IL-7 administration (supplemental Table 1), except for the single patient treated with 30 μg/kg of IL-7 from whom the sample (indicated by the asterisk) was obtained at 24 hours. (B) Expression of α4β7 in naive and memory CD4+ T cells from 2 representative individuals before and after treatment with a low (Patient 4; 3 μg/kg) or high (Patient 11; 60 μg/kg) dose of IL-7. The shaded histograms denote the staining in posttreatment samples; the empty profiles denote the staining in pretreatment samples.
In vivo treatment with IL-7 induces α4β7 in humans. (A) Expression of α4β7, CXCR4, CD127, CD95, and CD25 as analyzed by flow cytometry in circulating T cells derived from HIV-1–infected individuals under antiretroviral therapy before and after in vivo injection of the indicated doses of IL-7 or placebo. Naive and memory CD4+ and CD8+ T-cell populations were separately analyzed by multicolor staining and individual gating. For all markers except CD25, each dot represents the percentage increase in expression (MFI) from baseline after the indicated treatment for each of the subjects studied; for CD25 (for which MFI values could be misleading because of a marked shift in fluorescence intensity restricted to a minor subpopulation of cells), the percentage of positive cells is presented. Posttreatment samples were obtained on days 4, 7, or 14 after IL-7 administration (supplemental Table 1), except for the single patient treated with 30 μg/kg of IL-7 from whom the sample (indicated by the asterisk) was obtained at 24 hours. (B) Expression of α4β7 in naive and memory CD4+ T cells from 2 representative individuals before and after treatment with a low (Patient 4; 3 μg/kg) or high (Patient 11; 60 μg/kg) dose of IL-7. The shaded histograms denote the staining in posttreatment samples; the empty profiles denote the staining in pretreatment samples.
Discussion
Although the role played by IL-7 in the immunologic reconstitution of lymphopenic hosts is well established,2 the molecular mechanisms underlying this process remain largely undefined. Our study provides evidence that IL-7, at suprahomeostatic concentrations compatible with those that promote T-cell reconstitution in lymphopenic hosts,29,30 is a potent inducer and activator of the gut-homing integrin α4β7 and effectively redirects naive T cells to the intestinal compartment. The physiologic relevance of these observations is further illustrated by the dose-dependent induction of α4β7 observed in peripheral blood T cells from patients receiving IL-7 in vivo, in whom circulating lymphocytes are known to rapidly extravasate toward peripheral tissues within few hours of IL-7 injection.7 These findings suggest that the gut-associated lymphoid tissue (GALT) may represent a critical anatomical site in the process of T-cell reconstitution in lymphopenic hosts. This hypothesis is supported by the observation that efficient T-cell reconstitution in lymphopenic hosts requires stimulation by peptides derived from the intestinal commensal microflora.2 Moreover, the intestinal tract may provide a favorable environment to support extensive T-cell proliferation without causing inflammatory side effects because of inherent mechanisms of immunologic tolerance.31 Thus, IL-7 would be the key factor promoting migration of naive T cells into the intestinal compartment where these cells can find critical stimulatory factors provided by the commensal microflora as well as an immunologically safe milieu to allow their homeostatic proliferation.
Previous studies identified RA as the primary inducer of α4β7 expression and gut homing in T cells.16,17 RA is a metabolite of vitamin A released by dendritic cells residing in mesenteric lymph nodes during the course of immune responses against intestinal pathogens.17 Accordingly, as confirmed in our study, α4β7 induction by RA requires concomitant TCR-mediated stimulation.17 In contrast, IL-7–mediated α4β7 induction does not require concomitant stimulations and is actually abrogated in TCR-stimulated T cells because of down-modulation of the IL-7 receptor. Thus, our results suggest that T-cell intestinal homing may occur by 2 alternative pathways, which reflect 2 distinct physiologic conditions: one, driven by IL-7, associated with a program of homeostatic response to lymphopenia and aimed at reconstituting the T-cell pool without a potentially harmful phenotypic switch of naive to memory T cells; the other, driven by RA and TCR stimulation, associated with an antigen-specific immune response and leading to the irreversible conversion of naive to memory T cells. This model is supported by the observation that IL-7 induces naive T cells to acquire a memory-like phenotype without losing their naive identity; furthermore, IL-7 triggers a cold proliferation, uncoupled from the expression of T-cell activation markers. Interestingly, a transient memory-like masquerade of naive T cells was previously documented during T-cell repopulation in lymphopenic mice,27,28 as well as in macaques treated with IL-7.8 Thus, our results are compatible with a new model of host response to lymphopenia, which involves intestinal homing and transient memory-like transformation of naive T cells as critical steps in the IL-7–driven reconstitution of the T-cell pool.
In contrast to naive T cells, the majority of memory T cells do not up-regulate α4β7 in response to IL-7. This phenomenon could not be ascribed to lack of expression and functionality of the IL-7 receptor, because most memory T cells are able to transduce intracellular signals and proliferate on IL-7 treatment. The lack of α4β7 induction in most memory T cells is consistent with the fact that these cells, unlike naive T cells, do not require signals provided by the intestinal commensal microflora to proliferate2 ; therefore, their repopulation in lymphopenic hosts can occur independently of gut homing. The nature and function of the small subset of memory T cells that up-regulate α4β7 in response to IL-7 treatment are presently unknown. It will be interesting to investigate the relation between these memory T cells and colitogenic memory CD4+ T cells, which depend on IL-7 for their survival,32 as well as with T helper type 17 (Th17) cells,33 another memory T-cell subset that depends on IL-7 for its survival and expansion and preferentially homes to the intestinal compartment.34 The ability of IL-7 to induce and activate α4β7 could be relevant to various clinical settings, including inflammatory bowel diseases and HIV-1 infection. Indeed, α4β7 has been identified as an additional receptor molecule for HIV-1,35 which might facilitate the initial transmission of the virus and its subsequent spread to lymphoid organs, particularly to the GALT.36 Thus, it is conceivable that α4β7 activation and proliferation induced by IL-7 could influence the levels of HIV-1 replication in the gut compartment. We are currently investigating the effects of the spontaneous rise in endogenous IL-7 and the consequent up-regulation of α4β7 during the progression toward full-blown AIDS.
Whereas the role of IL-7 in T-cell homeostasis appears to be conserved through mammalian evolution, we found that its capacity to induce α4β7 is not. Indeed, we demonstrated that mouse cells do not up-regulate α4β7 on IL-7 stimulation either in vitro or in vivo, suggesting that the ability of IL-7 to induce α4β7 has emerged only recently during evolution. Thus, as seen with other immunologic mechanisms including the role of IL-7 in B-cell development,2 our observations suggest that humans and mice have evolved different pathways to achieve T-cell homeostasis, emphasizing the differences between human and murine immunology.
In conclusion, the results of our study shed new light on the complex molecular mechanisms underlying the effects of IL-7 both in lymphopenic hosts and in patients receiving IL-7 treatment. These findings might be relevant to the clinical management of lymphopenic conditions and may provide insights into the development of novel therapeutic or preventive measures related to lymphopenia. In vivo studies in animal models will be important to address the relevance of IL-7–mediated gut homing of T cells in the immunologic reconstitution of lymphopenic hosts. Furthermore, the precise definition of the intracellular signaling pathways that lead to up-regulation of α4β7 in naive, but not in most memory T cells, may provide new therapeutic targets for the fine modulation of T-cell homeostasis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Huiyi Miao and Nikita Patel for technical help, Il-Young Hwang for help with the mouse studies, Michel Morre for recombinant macaque IL-7, Maria Letizia Giardino Torchia for helpful suggestions and critical reading of the manuscript, the NIH AIDS Research and Reference Reagent Program for mAb Act-1, and the NIH Blood Bank volunteer donors.
This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, NIH. The clinical trial ACTG5214 was sponsored by the AIDS Clinical Trials Group and funded by the NIH (grants AI 068636 and AI-069501).
National Institutes of Health
Authorship
Contribution: R.C. designed the study, performed experiments, analyzed and interpreted data, and wrote the paper; L.V. designed and performed experiments, and analyzed and interpreted data; J.A. contributed to study design; C.C. contributed to study design; J.H.K. designed experiments; C.P. performed experiments; I.S. recruited, treated, and monitored patients; M.M.L. recruited, treated, and monitored patients; A.S.F. interpreted data and wrote the paper; and P.L. designed the study, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: M.M.L. has a clinical treatment trial contract with Cytheris. The remaining authors declare no competing financial interests.
The current affiliation for L.V. is Division of Immunoinfectivology, Children's Hospital Bambin Gesú, University of Rome “Tor Vergata,” Roma, Italy.
Correspondence: Paolo Lusso, National Institutes of Health, 10 Center Dr, Bldg 10, Rm 6A11, Bethesda, MD 20892; e-mail: plusso@niaid.nih.gov.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal