Abstract
In multiple myeloma, c-MYC is activated and contributes to the malignant phenotype. Targeting MYC by short hairpin RNA induced cell death in myeloma cell lines; however, cell lines are generated from samples taken in advanced stages of the disease and may not reflect patient cells adequately. In this study, we used the selective small molecule inhibitor of MYC-MAX heterodimerization, 10058-F4, on myeloma cell lines as well as primary myeloma cells, and we show that inhibition of c-MYC activity efficiently induces myeloma cell death. Moreover, in cocultures of cell lines with bone marrow stromal cells from myeloma patients, the inhibitor still induces apoptosis. Our results provide further evidence that myeloma cells are addicted to c-MYC activity and that c-MYC is a promising therapeutic target in multiple myeloma.
Introduction
Members of the MYC family are important oncogenes involved in the development of malignant cells.1 This may also be the case in multiple myeloma (MM), a malignancy of antibody-producing plasma cells in bone marrow. The activity of c-MYC in MM increases with disease stage.2,3 The mechanism by which c-MYC is activated in each case is unclear; however, multiple signaling pathways converge on c-MYC. Translocations involving MYC and immunoglobulin genes (IG) are relatively rare in MM and considered late progression events.4 c-MYC regulates transcription of up to 15% of the genes in human cells by binding to its obligate partner MAX. Many cancer cells may develop a dependency on c-MYC activity; and by preventing this activity, the cells may stop dividing or even undergo apoptosis. In agreement with this, short-hairpin RNA targeting MYC was shown to be lethal to a number of human myeloma cell lines.5 A small-molecule inhibitor, termed 10058-F4, has been identified that is proposed to specifically inhibit c-MYC–MAX heterodimerization, thereby preventing transactivation of c-MYC target genes.6,7 The inhibitor has been shown to have growth inhibitory effects on lymphoma and acute myelogenous leukemia cells.8,9
Methods
Cells
Myeloma cell lines used in this study were U266, INA-6, JJN-3, KMS-12-BM, IH-1, and KJON. Details on cell culture conditions are found in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). CD138+ patient cells obtained through the Norwegian Myeloma Biobank were purified using RoboSep automated cell separator and Human CD138 Positive Selection Kit (Stem Cell Technologies). Bone marrow stromal cells (BMSCs) from patients were obtained by plastic adherence and cultivated as stated in supplemental Methods. The project was approved by the Regional Ethics Committee, and patients gave informed consent in accordance with the Declaration of Helsinki. Patient characteristics are described in supplemental Table 1.
Cell viability measurements and quantitative RT-PCR were performed as described previously.10 PCR TaqMan assays used were as follows: MYC, Hs00153408_m1; MYCL1, Hs00420495_m1; and GAPDH, Hs99999905_m1 (Applied Biosystems).
Description of other reagents, immunoblotting, and knockdown experiments is found in supplemental Methods.
Results and discussion
To address the c-MYC dependency of myeloma cells, we decided to evaluate the effect of 10058-F4 in myeloma cell lines and primary cells by in vitro studies. First, both mRNA and protein expression of c-MYC and L-MYC was determined in 6 cell lines by quantitative RT-PCR and immunoblotting (Figure 1A). Five of the cell lines expressed c-MYC, whereas U266 only had L-MYC, as previously reported.11 L-MYC mRNA was also detected in KMS-12-BM and to a lesser extent in INA-6 and JJN-3, although the levels of L-MYC were negligible compared with c-MYC. The effect on cell viability was evaluated in myeloma cell lines treated with increasing concentrations of 10058-F4 for 48 hours. Apoptosis was induced by 50-100μM 10058-F4 in all cell lines expressing c-MYC, whereas the U266 cell line, which does not express c-MYC, was only minimally sensitive at 100μM (Figure 1B). This finding is consistent with the proposal that 10058-F4 is a specific inhibitor of c-MYC–MAX heterodimerization at concentrations < 100μM.7,8 Forced expression of L-MYC12 in INA-6 cells did not counteract apoptosis induced by 10058-F4 (supplemental Figure 2). Thus, even though c-MYC and L-MYC share some properties, it is not obvious that L-MYC simply can replace c-MYC in survival of myeloma cells. Interestingly, the 2 cell lines in the panel harboring IG-MYC translocations,4 JJN-3 and KMS-12-BM, expressed the highest amounts of c-MYC and were less sensitive to the inhibitor than the remaining 3 cell lines. We further measured mRNA expression of MYC and MYCL1 by quantitative RT-PCR in primary myeloma cells purified from bone marrow aspirates (n = 10) or peripheral blood (n = 1; for patient characteristics, see supplemental Table 1). All patient cells tested expressed MYC mRNA at similar or higher levels than the cell lines (Figure 1C). Next, the effect of the inhibitor on cell viability was evaluated (Figure 1D). The cells were incubated with 10058-F4 for 72 hours before staining and analysis as described in Figure 1B. All cells tested were sensitive to the inhibitor, but to various degrees. MM4, MM5, MM7, MM8, and MM10 all had IC50 < 50μM, whereas the rest of the cell samples had IC50 > 50 and < 100μM. The least sensitive cells were from patient MM1. These cells also expressed the highest amount of MYC mRNA of the samples tested, suggesting that more inhibitor may be needed when the cells express relatively high amounts of MYC. For clarity, a comparison between the relative MYC RQ values and the IC50 of 10058-F4 is provided in supplemental Table 2.
The 10058-F4 induced apoptosis in c-MYC–expressing primary myeloma cells and myeloma cell lines irrespective of IG-MYC translocations. (A) Expression of c-MYC and L-MYC mRNA and protein was evaluated in 6 human myeloma cell lines by quantitative RT-PCR and immunoblotting. The relative quantification (RQ) of mRNA levels was calculated using the δ δ Ct method with GAPDH as housekeeping gene. All mRNA values were compared with MYC mRNA in the KJON cell line, which was set to 1. (B) The effect on cell viability was evaluated by annexin-V FITC and propidium iodide staining after treatment by 10058-F4 for 48 hours. Examples of flow cytometry dot-plots are found in supplemental Figure 1. (C) Expression of MYC mRNA in 11 CD138+ primary myeloma cells. Levels of MYC in IH-1 and KMS-12-BM cell lines are included for comparison. For patient MM8, cDNA was not available. (D) Cell viability of 12 patient samples treated for 72 hours with increasing amounts of 10058-F4. Error bars represent SD of triplicate (A,C) or duplicate measurements (B,D).
The 10058-F4 induced apoptosis in c-MYC–expressing primary myeloma cells and myeloma cell lines irrespective of IG-MYC translocations. (A) Expression of c-MYC and L-MYC mRNA and protein was evaluated in 6 human myeloma cell lines by quantitative RT-PCR and immunoblotting. The relative quantification (RQ) of mRNA levels was calculated using the δ δ Ct method with GAPDH as housekeeping gene. All mRNA values were compared with MYC mRNA in the KJON cell line, which was set to 1. (B) The effect on cell viability was evaluated by annexin-V FITC and propidium iodide staining after treatment by 10058-F4 for 48 hours. Examples of flow cytometry dot-plots are found in supplemental Figure 1. (C) Expression of MYC mRNA in 11 CD138+ primary myeloma cells. Levels of MYC in IH-1 and KMS-12-BM cell lines are included for comparison. For patient MM8, cDNA was not available. (D) Cell viability of 12 patient samples treated for 72 hours with increasing amounts of 10058-F4. Error bars represent SD of triplicate (A,C) or duplicate measurements (B,D).
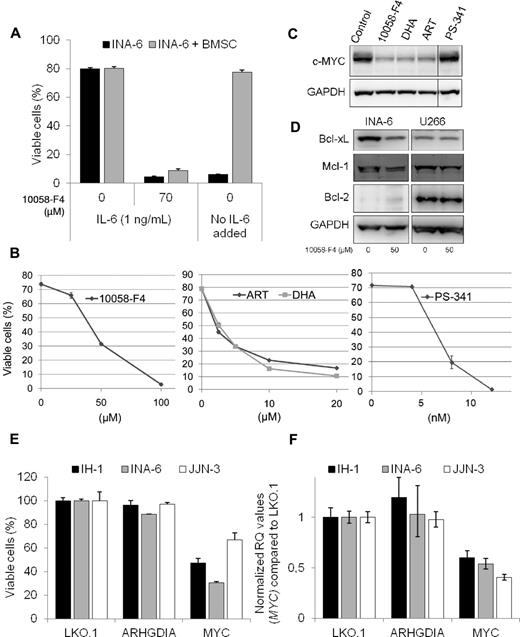
The 10058-F4 is not applicable in vivo,13 but it is possible to test effects of the inhibitor in cocultures of myeloma cells and BMSCs. INA-6 cells were added to a monolayer of BMSCs derived from a multiple myeloma patient and treated with 10058-F4 for 3 days before analysis of cell viability. The BMSCs were labeled with the cytosolic dye CFDA-SE before INA-6 cells were added to the culture, making it possible to discriminate between the 2 cell types. INA-6 cells were equally sensitive to apoptosis induced by 10058-F4 in the presence and absence of MM-derived BMSCs (Figure 2A). In contrast, BMSCs protected INA-6 cells from apoptosis induced by IL-6 deprivation, as previously shown.14
The 10058-F4 induced apoptosis in INA-6 cells is not prevented by BMSCs and is associated with down-regulation of MYC and antiapoptotic Bcl-2 proteins. (A) INA-6 cells were grown in 2% human serum in RPMI, with or without IL-6 (1 ng/mL) or 10058-F4, either alone or in the presence of a monolayer of BMSCs derived from an MM patient. The stromal cells were labeled with CFDA-SE (1μM) before INA-6 cells were added to the wells. After 72 hours, cells were detached by Accutase treatment, labeled with annexin-V Alexa-647, and analyzed by flow cytometry. INA-6 cells that were both CFDA-SE and annexin-V negative were considered viable. Error bars represent SD of triplicate measurements. (B) INA-6 cells were treated for 48 hours with the indicated concentrations of 10058-F4, DHA, ART, or PS-341 before measurement of cell viability. Cells were analyzed by flow cytometry, and cells that were both annexin-V FITC- and PI-negative were considered viable. Error bars represent SD of duplicate measurements. (C) Expression of c-MYC or GAPDH protein levels in INA-6 cells treated for 20 hours with 10058-F4 (50μM), DHA (10μM), ART (10μM), or PS-341 (8nM). (D) Immunoblot showing INA-6 and U266 after treatment for 20 hours with 10058-F4 as indicated. Protein levels of Bcl-xL, Mcl-1, Bcl-2, and GAPDH were measured using specific antibodies. (E) Myeloma cell lines were treated with the indicated lentiviral short hairpin RNA particles, and cell viability was evaluated after 3 days. (F) Relative MYC mRNA levels in myeloma cell lines treated for 2 days with the indicated lentiviral short hairpin RNA particles. The relative quantification (RQ) of mRNA levels was calculated using the δ δ Ct method with GAPDH as housekeeping gene. The MYC mRNA values were set to 1 in the LKO.1-transduced control cells.
The 10058-F4 induced apoptosis in INA-6 cells is not prevented by BMSCs and is associated with down-regulation of MYC and antiapoptotic Bcl-2 proteins. (A) INA-6 cells were grown in 2% human serum in RPMI, with or without IL-6 (1 ng/mL) or 10058-F4, either alone or in the presence of a monolayer of BMSCs derived from an MM patient. The stromal cells were labeled with CFDA-SE (1μM) before INA-6 cells were added to the wells. After 72 hours, cells were detached by Accutase treatment, labeled with annexin-V Alexa-647, and analyzed by flow cytometry. INA-6 cells that were both CFDA-SE and annexin-V negative were considered viable. Error bars represent SD of triplicate measurements. (B) INA-6 cells were treated for 48 hours with the indicated concentrations of 10058-F4, DHA, ART, or PS-341 before measurement of cell viability. Cells were analyzed by flow cytometry, and cells that were both annexin-V FITC- and PI-negative were considered viable. Error bars represent SD of duplicate measurements. (C) Expression of c-MYC or GAPDH protein levels in INA-6 cells treated for 20 hours with 10058-F4 (50μM), DHA (10μM), ART (10μM), or PS-341 (8nM). (D) Immunoblot showing INA-6 and U266 after treatment for 20 hours with 10058-F4 as indicated. Protein levels of Bcl-xL, Mcl-1, Bcl-2, and GAPDH were measured using specific antibodies. (E) Myeloma cell lines were treated with the indicated lentiviral short hairpin RNA particles, and cell viability was evaluated after 3 days. (F) Relative MYC mRNA levels in myeloma cell lines treated for 2 days with the indicated lentiviral short hairpin RNA particles. The relative quantification (RQ) of mRNA levels was calculated using the δ δ Ct method with GAPDH as housekeeping gene. The MYC mRNA values were set to 1 in the LKO.1-transduced control cells.
To further correlate inhibition of c-MYC to apoptosis in myeloma cells, we tested other compounds reported to inhibit or down-regulate c-MYC. The antimalaria drugs artesunate (ART) and dihydroartemisinin (DHA) have been reported to down-regulate c-MYC.15 In addition, in MM cells, treatment with ART or DHA led to induction of apoptosis concomitant with down-regulation of c-MYC protein (Figure 2B-C). In contrast, lethal doses of PS-341 (bortezomib) did not decrease c-MYC expression (Figure 2B-C).
To elucidate the mechanisms involved in apoptosis by the c-MYC inhibitor in MM cells, protein levels of the antiapoptotic Bcl-2 family members Bcl-xL, Mcl-1, and Bcl-2 were measured in INA-6 and U266 after treatment with 10058-F4 for 20 hours (Figure 2D). Bcl-xL expression was down-regulated by 10058-F4 in INA-6, but not in the U266 cell line. The effect on Mcl-1 protein expression was minor. Bcl-2 was not detected in INA-6 by immunoblotting, which is in accordance with previous findings.16 In U266, protein levels of Bcl-2 were clearly detectable but did not change on treatment with the c-MYC inhibitor.
Finally, we treated INA-6, IH-1, and JJN-3 cells with lentiviral short hairpin RNA particles targeting MYC and measured MYC mRNA levels after 2 days and cell viability after 3 days (Figure 2E-F). Knockdown of MYC lead to decreased viability in all 3 cell lines, in line with previous findings in cell lines5 and with published data on inhibition of c-MYC using a BET family protein inhibitor, JQ1, that led to a significant reduction of viability in primary cells.17
Our findings further support the idea that inhibition of c-MYC activity by targeting MYC-MAX heterodimerization may be lethal to most myeloma cells. Thus, c-MYC should be an attractive target for therapy of MM.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Lill Anny Gunnes Grøseth and Berit Fladvad Størdal for expert technical assistance, the Norwegian Myeloma Biobank for patient samples, and Karin Fahl Wader for valuable help organizing clinical data from patients.
This work was supported by the Norwegian Cancer Society and the Norwegian Research Council.
Authorship
Contribution: T.H., T.K.V., H.H., and A.S. designed the study and performed analyses; A.W. provided patient material; and T.H. and A.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Toril Holien, Department of Cancer Research and Molecular Medicine, Norwegian University of Science and Technology, Gastrosenter 3. etg Sør, St Olavs Hospital, Prinsesse Kristinas gate 1, Trondheim N-7006, Norway; e-mail: toril.holien@ntnu.no.