Abstract
Serum response factor and its transcriptional cofactor MKL1 are critical for megakaryocyte maturation and platelet formation. We show that MKL2, a homologue of MKL1, is expressed in megakaryocytes and plays a role in megakaryocyte maturation. Using a megakaryocyte-specific Mkl2 knockout (KO) mouse on the conventional Mkl1 KO background to produce double KO (DKO) megakaryocytes and platelets, a critical role for MKL2 is revealed. The decrease in megakaryocyte ploidy and platelet counts of DKO mice is more severe than in Mkl1 KO mice. Platelet dysfunction in DKO mice is revealed by prolonged bleeding times and ineffective platelet activation in vitro in response to adenosine 5′-diphosphate. Electron microscopy and immunofluorescence of DKO megakaryocytes and platelets indicate abnormal cytoskeletal and membrane organization with decreased granule complexity. Surprisingly, the DKO mice have a more extreme thrombocytopenia than mice lacking serum response factor (SRF) expression in the megakaryocyte compartment. Comparison of gene expression reveals approximately 4400 genes whose expression is differentially affected in DKO compared with megakaryocytes deficient in SRF, strongly suggesting that MKL1 and MKL2 have both SRF-dependent and SRF-independent activity in megakaryocytopoiesis.
Introduction
Biphenotypic megakaryocyte-erythroid precursors undergo differentiation and endomitosis to become mature polyploid megakaryocytes that release platelets into the circulation. Despite advances in our understanding of hematopoiesis, much remains unknown regarding the regulation of megakaryocytopoiesis. Our laboratory is focused on understanding the mechanisms of normal megakaryocyte differentiation to better understand acute megakaryoblastic leukemia (AMKL). The reciprocal t(1;22) translocation that is consistently associated with AMKL results in fusion of the RBM15 (RNA-binding motif 15) and MKL1 (megakaryoblastic leukemia 1) genetic loci.1,2 RBM15, an RNA-binding protein whose function is not yet well defined, is differentially expressed in hematopoiesis with the highest mRNA levels in progenitors and lowest in differentiated blood cells.3 Here, we focus on the role of the MKL1 family of proteins in megakaryocytopoiesis.
MKL1 (MRTF-A, MAL, BSAC) is a transcriptional cofactor belonging to the myocardin-related transcription factor (MRTFs) family that also includes myocardin and MKL2 (MRTF-B, MAL16). These proteins function through association with serum response factor (SRF), a ubiquitously expressed transcription factor.4 SRF is also activated by association with ternary complex factors (Elk1, SAP1, SAP2).5 Transcriptional cofactor binding determines the spatiotemporal activity of SRF. Srf knockout (KO) mice die early in gestation because of abnormal mesoderm development.6 Myocardin and Mkl2 KO mice are also embryonic lethal because of abnormal cardiac development.7-9 Mkl1 KO mice show partial embryonic lethality, with some embryos dying because of myocardial cell necrosis. However, Mkl1 KO mice that complete gestation have normal lifespans. Functions for MKL1 have been characterized in embryonic stem cells, fibroblasts, smooth muscle cells, and neurons.10-12
Recently, we and others characterized the hematopoietic phenotypes of Mkl1 KO and megakaryocyte-specific Srf conditional KO (Srf Pf4-cKO) mice.13-15 In each case, mice have thrombocytopenia with increased numbers of immature megakaryocytes in the bone marrow (BM) suggesting both a failure of normal megakaryocyte maturation as well as abnormal formation of the megakaryocyte and platelet cytoskeleton. Comparison of the Mkl1 KO and Srf Pf4-cKO mice as well as in vitro cell-culture studies suggest that the effect of MKL1 in megakaryocyte differentiation is mediated by association with SRF.13 However, the hematopoietic phenotype is far more severe in Srf Pf4-cKO than Mkl1 KO mice, which indicates that other factors may act in conjunction with, or in the absence of, MKL1 to promote SRF-mediated megakaryocyte maturation. Possible contributing proteins include other members of the MRTF family.
Here, we assessed the contribution of MKL2 to megakaryocytopoiesis, and found that while it is not required for effective megakaryocyte maturation and platelet formation, MKL2 compensates for and mitigates the effects of MKL1 deficiency. Mice lacking both MKL1 and MKL2 in the megakaryocyte lineage have macrothrombocytopenia as well as platelet cytoskeletal abnormalities and severely impaired platelet activation. In addition to the peripheral blood defects, there is abnormal megakaryocyte ultrastructure. Surprisingly, the phenotype of double KO (DKO) mice is more extreme than that of the Srf Pf4-cKO mice, strongly suggesting that MRTFs function in ways that are independent of their SRF transcriptional coactivation activities.
Methods
Mouse strains
All procedures were performed in compliance with relevant laws and institutional guidelines and were approved by the Yale University Institutional Animal Care and Use Committee. Mkl2F/F mice16 were crossed with platelet factor 4 (Pf4) Cre17 -expressing mice. These mice were then bred onto the Mkl1 KO background.7 Mkl1 KO and Pf4-Cre mice crossed with SrfF/F were as previously described.13,14 All mice were on a C57Bl/6J background.
Flow cytometry, cell sorting, and in vitro culture
To determine mouse BM progenitor populations, freshly isolated BM was incubated with anti-CD16/CD32 antibodies (Fc block; BD Biosciences), stained with PE-biotin lineage detection cocktail (Miltenyi Biotec), allophycocyanin-H7 CD117/c-kit (BD Biosciences), Alexa 647 Sca-1, PE-Cy5 CD150, PE-Cy7 CD105 (BioLegend), and FITC CD41 (BD Biosciences), and analyzed (LSRII; BD Biosciences) or sorted (FACS Aria, BD Biosciences; or MoFlo, Beckman Coulter) as previously described.18 For sorting experiments, BM was first enriched for stem/progenitor cells using immunomagnetic separation (BD Biosciences). LSK (lineage [lin] negative, CD117 positive, Sca-1 positive), PreMegE (lin negative, CD117 positive, CD41 negative, CD150 positive, CD105 negative), and MkP (lin negative, CD117 positive, CD41 positive) were assayed. For DNA content analysis, unfractionated whole BM was stained with FITC CD41, then treated with 70% ethanol overnight followed by digestion with 20 μg/mL RNase (Sigma-Aldrich) on ice for 4 hours. Cells were resuspended with 10 μg/mL propidium iodide (Sigma-Aldrich) before analysis on a FACSCalibur (BD Biosciences). Platelet activation was assessed using FITC CD41/61 and PE JON/A (Emfret Analytics). Flow cytometric data were analyzed using FlowJo software (TreeStar). PreMegE and MkP were differentiated for 5 or 3 days, respectively, in megakaryocyte differentiation medium containing StemSpan Serum Free Expansion Media (StemCell Technologies) supplemented with 30% BIT 9500, l-glutamine (Life Technologies), penicillin/streptomycin (P/S; Life Technologies), and 50 ng/mL murine thrombopoietin (mTPO; ConnStem). Fetal liver megakaryocytes were obtained from E13.5 embryos and cultured for 4 days in low-glucose DMEM (Life Technologies), 10% fetal bovine serum (Gemini), P/S, and 50 ng/mL mTPO.
Platelet preparations
Peripheral blood was collected from the retro-orbital sinus into tubes containing acid citrate dextrose (ACD) anticoagulant. Platelet-rich plasma was prepared as previously described.19 For flow cytometry, 50 μL of whole blood was added to 200 μL of 20 U/mL heparin in Tris-buffered saline (20mM Tris-HCl, 137mM NaCl). After further dilution with 1 mL of 2mM CaCl2 in modified Tyrode-HEPES buffer (5mM HEPES, 140mM NaCl, 2.7mM KCl, 5.5mM dextrose, 0.42mM Na2HPO4, 12mM NaHCO3), platelets were stimulated with 1mM adenosine 5′-diphosphate (ADP; Sigma-Aldrich).
Immunofluorescence
Platelets isolated from peripheral blood or fetal liver–derived megakaryocytes were spun onto poly-L-lysine–coated coverslips and either immediately fixed with 2% paraformaldehyde or allowed to spread for 20 minutes and then fixed (platelets only). Permeabilization, blocking, and staining with using a β1 tubulin antibody (Genemed Synthesis Inc) and Alexa Fluor 568 Phalloidin (Life Technologies) were conducted as previously described.20 Samples were examined with an Axiovert 200 microscope (Carl Zeiss Inc) equipped with a 63× NA 1.4 oil-immersion objective. Images were obtained using a charge-coupled device camera (Hamamatsu Photonics) and phalloidin quantified using NIH ImageJ software.
DNA and RNA analysis
DNA and RNA were extracted using the QIAGEN DNeasy Blood and Tissue and Ambion RNAqueous Micro kits, respectively. cDNA was made using Superscript III (Life Technologies) with random primers (Life Technologies). Quantification was performed using a CFX96 C1000 thermal cycler (Bio-Rad) using TaqMan gene expression assays (Applied Biosystems–Life Technologies): murine epidermal growth factor-like domain 7 (EGFL7) and murine Spred1, and eukaryotic 18S as an internal control. U6 RNA was used as an internal control for miR-126 as assayed by QIAGEN miScript SYBR Green primer assay. MkP were sorted from the BM of 5- to 10-week-old mice and RNA harvested after cells were differentiated for 3 days in megakaryocyte differentiation media. Samples were collected from at least 8 different mice for each genotype and pooled for RNA sequencing. Library preparation and sequencing were performed by the Yale Stem Cell Genomics Core Facility using the Illumina TruSeq RNA Sample Preparation kit. Samples were sequenced on an Illumina HiSeq 2000 using 50-cycle single-end sequencing. FASTQ format sequencing reads were aligned to the mm9 genome using Tophat Version 1.3.1 software.21 The cufflinks, cuffmerge, and cuffdiff Version 1.3.0 programs were used to identify differences in Ensembl transcripts.22 The analysis used upper-quartile normalization, multiread, and GC fragment bias corrections, and masking of reads in rRNA and tRNA genes. Sample comparisons are displayed using the R heatmap.2, lumi, and VennDiagram packages. Data are publicly available through Gene Expression Omnibus (file numbers pending). Statistically significant differences between genotypes were defined by genes having more than 10 fragments per kilobase of transcript per million (FPKM) reads in at least 1 of the 2 samples compared and a q value of less than 0.05.
Bone marrow histology
Femurs were fixed overnight in 4% paraformaldehyde, decalcified in Decalcifier I solution (Surgipath) overnight, and transferred to 70% ethanol for processing by the Research Histology Facility at Yale School of Medicine for 5-μm longitudinal paraffin sections, hematoxylin and eosin (H&E) staining, and immunohistochemistry for von Willebrand factor (anti-VWF; DAKO).
Bleeding time measurements
Three-week-old mice were anesthetized using isoflurane (Butler Animal Health Support). Using a sharp razor blade, 0.5 cm of the tail was removed and the tail held in warm PBS. Bleeding time was measured as the time until bleeding stopped.
Statistical analysis
Statistical significance was assessed using the 2-tailed unpaired t test with Prism software (GraphPad).
Results
MKL2 is expressed in murine megakaryocytes
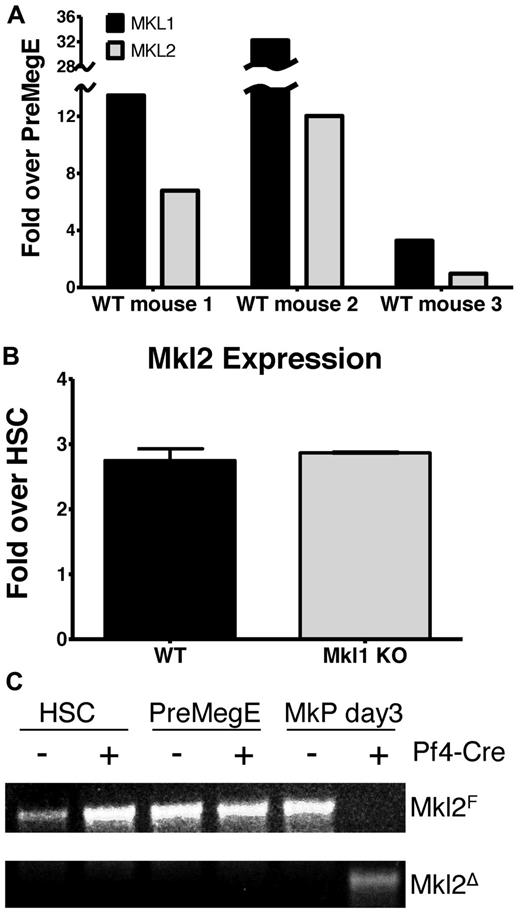
The hematopoietic phenotype of megakaryocyte-specific (Pf4-Cre) Srf conditional KO (Srf Pf4-cKO) is more severe than that of Mkl1 KO mice,14 suggesting compensation or redundancy in Mkl1 KO mice. Because there are multiple transcriptional coactivators of SRF, multiple other proteins may mitigate the effects of MKL1 deficiency. Possible genes that could compensate for the loss of MKL1 are Myocardin and Mkl2. Myocardin's expression is restricted to the heart. We investigated whether MKL2, known to be expressed in multiple cell types, is expressed in megakaryocytes. RNA was taken from PreMegE cells sorted by flow cytometry immediately postsort (day 0) and after culturing 5 days in megakaryocyte differentiation media. MKL2 expression increased during megakaryocyte differentiation, although not as dramatically as MKL1 (Figure 1A). We hypothesized that Mkl2 mRNA would be up-regulated to compensate for the lack of MKL1 in the Mkl1 KO mice. However, there was no increase in Mkl2 expression in megakaryocytes derived from PreMegE cells in Mkl1 KO mice compared with WT controls (Figure 1B).
MKL2 gene expression and validation of conditional Mkl2 KO mice. (A) MKL1 and MKL2 mRNA levels were assessed in PreMegE cells from 3 WT mice differentiated in vitro using megakaryocyte differentiation medium. Shown is the fold increase in mRNA over freshly sorted PreMegE of megakaryocytes from 5-day cultured PreMegE cells after normalization to the 18S internal control. All 3 mice show an increase in both MKL1 and MKL2 during megakaryocyte differentiation. (B) Mkl2 expression was assessed in megakaryocytes differentiated in vitro from PreMegE of WT (n = 3) and Mkl1 KO (n = 3) mice. Shown is the fold increase in mRNA over HSC. Error bars represent SEM. (C) PCR of genomic DNA isolated from HSC, PreMegE, and MkP after 3 days of mTPO culture showed specific deletion of the Mkl2 locus in megakaryocytes of Pf4-Cre expressing Mkl2F/F mice. Mkl2F/F mice without Pf4-Cre were negative controls.
MKL2 gene expression and validation of conditional Mkl2 KO mice. (A) MKL1 and MKL2 mRNA levels were assessed in PreMegE cells from 3 WT mice differentiated in vitro using megakaryocyte differentiation medium. Shown is the fold increase in mRNA over freshly sorted PreMegE of megakaryocytes from 5-day cultured PreMegE cells after normalization to the 18S internal control. All 3 mice show an increase in both MKL1 and MKL2 during megakaryocyte differentiation. (B) Mkl2 expression was assessed in megakaryocytes differentiated in vitro from PreMegE of WT (n = 3) and Mkl1 KO (n = 3) mice. Shown is the fold increase in mRNA over HSC. Error bars represent SEM. (C) PCR of genomic DNA isolated from HSC, PreMegE, and MkP after 3 days of mTPO culture showed specific deletion of the Mkl2 locus in megakaryocytes of Pf4-Cre expressing Mkl2F/F mice. Mkl2F/F mice without Pf4-Cre were negative controls.
Confirmation of Mkl2 conditional knockout mice in the megakaryocyte lineage
To assess the function of MKL2 in megakaryocytes, mice with megakaryocyte-specific KO of MKL2 (Mkl2 Pf4-cKO) were made by crossing Mkl2F/F mice with platelet factor 4 (Pf4)–Cre mice. In the Mkl2F/F mouse created by the Olson laboratory,16 the floxed region of the Mkl2 gene includes exon 8, which encodes the SRF-binding domain. Mkl2 Pf4-cKO mice are viable. Megakaryocyte-specific deletion was confirmed by genomic PCR of 3 cell populations: MkP cultured for 3 days in megakaryocyte differentiation media, freshly isolated hematopoietic stem/progenitor cells (LSK), and PreMegE (Figure 1C). As expected, deletion of Mkl2 was not detected in LSK or PreMegE populations, neither of which has yet committed to the megakaryocytic lineage. In contrast, the genome of the differentiated MkP from Pf4-Cre mice contained copies of the excised allele and not the floxed allele (Figure 1C).
Conditional DKO mice have thrombocytopenia with defective platelet activation
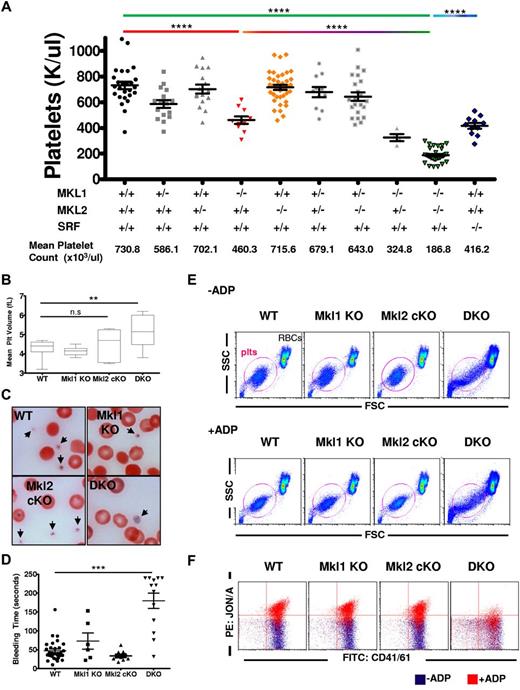
The Mkl2 Pf4-cKO mice were mated onto the Mkl1 KO background (referred to as “DKO mice”) to create DKO megakaryocytes. The DKO mice have macrothrombocytopenia; platelet counts of DKO mice are significantly (P < .0001) decreased (187 000 ± 56 000 platelets/μL) compared with WT (730 000 ± 148 000 platelets/μL), Mkl1 KO (460 000 ± 94 000 platelets/μL), and Mkl2 Pf4-cKO (715 000 ± 120 000 platelets/μL; Figure 2A). In the absence of MKL2, 1 copy of MKL1 is sufficient to maintain normal platelet counts (643 000 ± 157 000 platelets/μL). In contrast, mice with 1 copy of MKL2 in the absence of MKL1 have platelet counts (325 000 ± 54 000 platelets/μL) significantly higher than DKO mice, but lower than WT mice. Srf Pf4-cKO platelet numbers (416 000 ± 74 000 platelets/μL) were decreased compared with WT, consistent with previous reports,14 however, they were significantly higher than DKO mice. DKO mice also had increased mean platelet volume (MPV; Figure 2B) as assessed by peripheral blood smears (Figure 2C).
DKO mice have macrothrombocytopenia and dysfunctional platelets. Peripheral blood was taken from mice with the indicated genotypes and (A) platelet counts and (B) platelet volume analyzed. (C) Representative peripheral blood smears stained with Wright Giemsa are consistent with low platelet count and high MPV in DKO mice. Images were taken using an oil-immersion 100× lens. Black arrows indicate platelets. (D) Bleeding times from mice with different genotypes (WT, n = 31; Mkl1 KO, n = 6; Mkl2 cKO, n = 10; DKO, n = 13). (E) Flow cytometry of peripheral blood platelets showing FSC vs SSC in the absence (top) and presence (bottom) of ADP. Note change in shape of platelet gate (circled in red) in response to ADP stimulation. Red blood cells (RBCs) are indicated. (F) Representative data showing total CD41/61 (x-axis) versus the activated JON/A conformation (y-axis) of CD41/CD61 in resting (blue) and ADP treated (red) platelets of 4- to 6-week-old mice. (n.s. indiates not significant; ****P < .0001; ***P < .001; **P < .01). All error bars represent SEM. Data from 4 independent experiments are summarized in Table 1.
DKO mice have macrothrombocytopenia and dysfunctional platelets. Peripheral blood was taken from mice with the indicated genotypes and (A) platelet counts and (B) platelet volume analyzed. (C) Representative peripheral blood smears stained with Wright Giemsa are consistent with low platelet count and high MPV in DKO mice. Images were taken using an oil-immersion 100× lens. Black arrows indicate platelets. (D) Bleeding times from mice with different genotypes (WT, n = 31; Mkl1 KO, n = 6; Mkl2 cKO, n = 10; DKO, n = 13). (E) Flow cytometry of peripheral blood platelets showing FSC vs SSC in the absence (top) and presence (bottom) of ADP. Note change in shape of platelet gate (circled in red) in response to ADP stimulation. Red blood cells (RBCs) are indicated. (F) Representative data showing total CD41/61 (x-axis) versus the activated JON/A conformation (y-axis) of CD41/CD61 in resting (blue) and ADP treated (red) platelets of 4- to 6-week-old mice. (n.s. indiates not significant; ****P < .0001; ***P < .001; **P < .01). All error bars represent SEM. Data from 4 independent experiments are summarized in Table 1.
Further characterization of the DKO animals indicated defective hemostasis. Bleeding times of the DKO mice (171 ± 86 seconds) were significantly increased over wild-type (WT; 35 ± 14 seconds), Mkl1 KO (73 ± 52 seconds), and Mkl2 Pf4-cKO (34 ± 13 seconds) mice (Figure 2D). It is not clear why 3 of 13 DKO mice had bleeding times within the normal range. The prolonged bleeding times of DKO mice could be because of decreased platelet counts and/or platelet dysfunction. In vitro platelet function was assayed by appearance of activated CD41/CD61 and morphologic changes in response to ADP. In the resting state, WT, Mkl1 KO, Mkl2 Pf4-cKO, and Srf Pf4-cKO platelets displayed normal forward scatter (FSC) and side scatter (SSC). In contrast, DKO platelet morphology was less uniform and the platelet and red cell populations were not well separated (Figure 2E, Table 1), consistent with increased MPV. After ADP treatment, there was an increase in activated CD41/61 heterodimer on the surface of WT, Mkl1 KO, and Mkl2 Pf4-cKO platelets using a PE-conjugated JON/A antibody (Figure 2F, Table 1). In contrast, DKO platelets did not respond to ADP; there was no conformational change of the CD41/61 heterodimer to allow for JON/A antibody binding. In addition, DKO platelets did not display any shape change within the FSC/SSC gate after ADP treatment.
Platelet activation flow cytometry parameters
| . | Resting . | ADP activated . | ||||||
|---|---|---|---|---|---|---|---|---|
| WT . | MKL1 KO . | MKL2 cKO . | DKO . | WT . | MKL1 KO . | MKL2 cKO . | DKO . | |
| Mean FSC | 28 ± .32 | 25 ± .84 | 29 ± .42 | 47 ± 3.9* | 30 ± .53 | 29 ± .46 | 33 ± 1.2 | 52 ± 4.9* |
| JON/A, MFI | 17 ± .4 | 16 ± .26 | 17 ± .25 | 20 ± 1.0* | 106 ± 16 | 104 ± 11 | 108 ± 48 | 30 ± 1.4* |
| . | Resting . | ADP activated . | ||||||
|---|---|---|---|---|---|---|---|---|
| WT . | MKL1 KO . | MKL2 cKO . | DKO . | WT . | MKL1 KO . | MKL2 cKO . | DKO . | |
| Mean FSC | 28 ± .32 | 25 ± .84 | 29 ± .42 | 47 ± 3.9* | 30 ± .53 | 29 ± .46 | 33 ± 1.2 | 52 ± 4.9* |
| JON/A, MFI | 17 ± .4 | 16 ± .26 | 17 ± .25 | 20 ± 1.0* | 106 ± 16 | 104 ± 11 | 108 ± 48 | 30 ± 1.4* |
Mean FSC and MFI of JON/A staining was averaged and the SD displayed (n = 4).
ADP indicates adenosine 5′-diphosphate; WT, wild type; KO, knockout; cKO, conditional knockout; DKO, double knockout; FSC, forward scatter; and MFI, mean fluorescence intensity.
P < .001.
DKO platelets have cytoskeleton abnormalities and atypical morphology
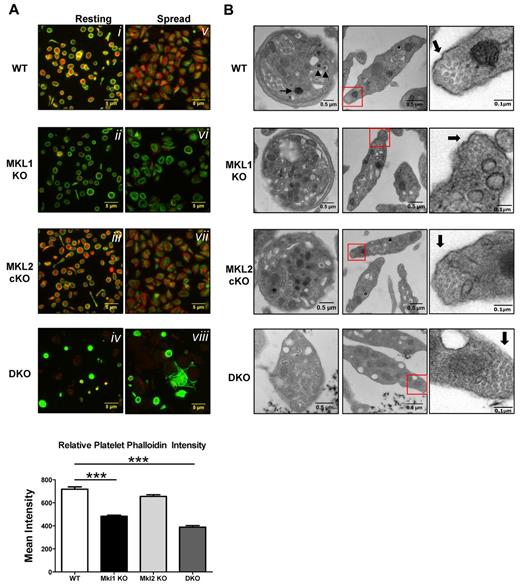
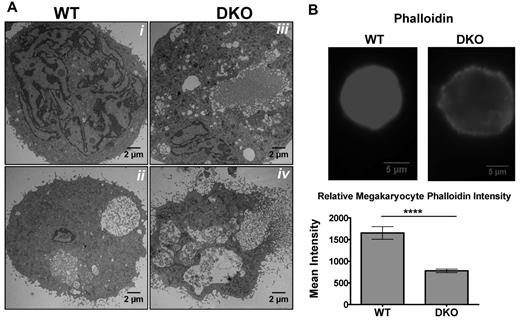
Flow cytometry suggested altered platelet structure and shape. Because many cytoskeleton genes are SRF targets, organization of polymerized actin and microtubules of the cytoskeleton were assessed. Immunofluorescence images of platelets, both resting and spread, showed differences in the actin and microtubule cytoskeleton organization in DKO platelets as assayed by phalloidin and β1-tubulin, a megakaryocyte lineage-specific isoform of tubulin (Figure 3A). Resting WT and Mkl2 Pf4-cKO platelets showed significant filamentous actin staining (shown in red) and distinct microtubule rings (shown in green) characteristic of platelets (Figure 3Ai,iii). Mkl1 KO platelets had an organized microtubule ring but lacked phalloidin staining (Figure 3Aii). After contact with glass, WT and Mkl2 Pf4-cKO platelets retract their tubulin coils, and polymerize actin to extend filopodia and lamellopodia, ultimately causing them to spread (Figure 3Av,vii). Although spread Mkl1 KO platelets had some microtubule reorganization, most retained partial microtubule coil morphology and the actin cytoskeleton remained disorganized (Figure 3Avi). DKO platelets did not change shape after adhesion to coverslips and had no filamentous actin staining (Figure 3Aviii). In addition to the abnormal platelet activation, DKO platelets were often amorphously shaped, unlike the characteristic discoid shape of WT platelets. Quantification of phalloidin by immunofluorescence confirmed the decrease in polymerized actin (Figure 3A bottom panels). Of the DKO platelets with detectable β1 tubulin, the marginal tubulin coil was thicker than their WT and single KO counterparts and revealed distinct heterogeneity in size and shape (Figure 3Aiv). The bright β1-tubulin immunofluorescence signal in the DKO platelets also suggested an increase in β1-tubulin protein.
DKO platelets lack normal cytoskeleton organization and granule complexity. Platelet-rich plasma was isolated from mouse blood. (A) Samples were spun onto poly-l-lysine–coated slides and fixed immediately or permitted to spread for 20 minutes on glass before fixation. Samples were permeabilized and probed for filamentous actin (red, phalloidin) and β1 tubulin (green). Quantification of phalloidin intensity by immunofluorescence showed decreased polymerized actin in Mkl1 KO and DKO platelets (bottom). Error bars represent SEM. (B) Thin-section electron micrographs highlight the heterogeneity in granule segregation and platelet morphology. DKO platelets lack α granules and their dense granules are not as opaque. Red squares in center panels indicate the magnified sections in the right panels. Magnified right panel images were modified for easier visualization of microtubule cross-sections by increasing contrast. Thick arrows indicate marginal band microtubules, which are increased in DKO mice (***P < .001).
DKO platelets lack normal cytoskeleton organization and granule complexity. Platelet-rich plasma was isolated from mouse blood. (A) Samples were spun onto poly-l-lysine–coated slides and fixed immediately or permitted to spread for 20 minutes on glass before fixation. Samples were permeabilized and probed for filamentous actin (red, phalloidin) and β1 tubulin (green). Quantification of phalloidin intensity by immunofluorescence showed decreased polymerized actin in Mkl1 KO and DKO platelets (bottom). Error bars represent SEM. (B) Thin-section electron micrographs highlight the heterogeneity in granule segregation and platelet morphology. DKO platelets lack α granules and their dense granules are not as opaque. Red squares in center panels indicate the magnified sections in the right panels. Magnified right panel images were modified for easier visualization of microtubule cross-sections by increasing contrast. Thick arrows indicate marginal band microtubules, which are increased in DKO mice (***P < .001).
As suggested by flow cytometry (Figure 2E), DKO platelets have less uniform granularity and size than WT, Mkl1 KO, and Mkl2 Pf4-cKO platelets. To analyze this defect in more detail, platelet ultrastructure was assessed by electron microscopy. The images shown highlight the defective cytoskeleton and abnormal granular contents of the DKO platelets (Figure 3B). A unique feature of platelets is the microtubule ring that lies just below the platelet surface. This ring is a single microtubule, looped 8-12 times, allowing a 100-micron tubule to exist within a 2- to 4-micron platelet, giving platelets their characteristic discoid shape. Electron micrograph analysis of WT, Mkl1 KO, and Mkl2 Pf4-cKO platelets revealed normal numbers of microtubule rings in cross-section8-12 at their tips while DKO platelets were seen to have more (up to 17) microtubules when cross-sectioned (Figure 3B thick black arrows). These data correlate with the increased intensity of the β1-tubulin immunostaining (Figure 3Aiv,viii). In general, the DKO platelets were amorphously shaped with poorly defined borders, unlike the uniformly discoid shape of the WT platelets. Most notable, however, was the loss of heterogeneity of the granular contents of the DKO platelets. Some of the DKO platelets had granular distribution very similar to WT, while others lacked both α and dense granules.
DKO BM has an accumulation of immature megakaryocytes
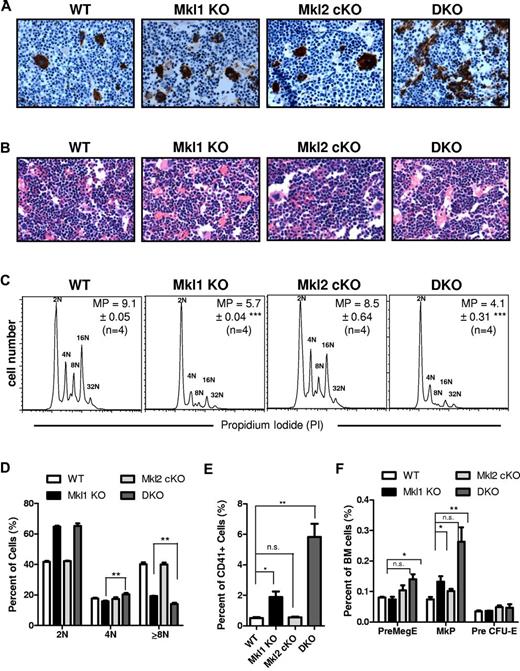
Platelet defects in peripheral blood suggest abnormal BM megakaryocytopoiesis. Immunohistochemistry for VWF on BM sections (Figure 4A) revealed an increase in megakaryocyte number in DKO, and, to a lesser extent, Mkl1 KO mice (already reported), compared with WT and Mkl2 Pf4-cKO BM. The VWF-positive megakaryocytes in the DKO were often small, with low ploidy and irregular cell morphology, consistent with the staining patterns found in Srf Pf4-cKO mice. H&E staining did not reveal any differences in BM cellularity (Figure 4B).
DKO mice have an accumulation of immature megakaryocytes in the BM. Paraffin sections from femurs of 6-week-old mice were stained with (A) anti-VWF antibody or (B) H&E. (C) Representative ploidy histograms for CD41+ bone marrow cells are shown along with the mean ploidy (MP) ± SEM of 4 mice per genotype. (D) Consistent with the decreased mean ploidy, the percentages of megakaryocytes with each ploidy level for n = 4 mice per genotype show that Mkl1 KO and DKO megakaryocytes have a significant increase in 2N megakaryocytes. (E) Flow cytometry confirms the increase in total CD41+ cells in the bone marrow using 4 mice per genotype. (F) Analysis of BM progenitors revealed an increase in the PreMegE and MkP populations in DKO BM (n.s. indicates not significant; **P < .01; *P < .05; all error bars represent SEM).
DKO mice have an accumulation of immature megakaryocytes in the BM. Paraffin sections from femurs of 6-week-old mice were stained with (A) anti-VWF antibody or (B) H&E. (C) Representative ploidy histograms for CD41+ bone marrow cells are shown along with the mean ploidy (MP) ± SEM of 4 mice per genotype. (D) Consistent with the decreased mean ploidy, the percentages of megakaryocytes with each ploidy level for n = 4 mice per genotype show that Mkl1 KO and DKO megakaryocytes have a significant increase in 2N megakaryocytes. (E) Flow cytometry confirms the increase in total CD41+ cells in the bone marrow using 4 mice per genotype. (F) Analysis of BM progenitors revealed an increase in the PreMegE and MkP populations in DKO BM (n.s. indicates not significant; **P < .01; *P < .05; all error bars represent SEM).
Flow cytometric analysis of DKO BM CD41+ cells showed a statistically significant (P < .01) shift to lower ploidy megakaryocytes compared with WT (Figure 4C-D). Ploidy measures the DNA content of cells, thus assessing the number of endomitotic cycles a cell has undergone. Mkl1 KO and DKO BM had a similar percentage of megakaryocytes that were 2N. However, there were fewer DKO megakaryocytes with high ploidy (≥ 8N) compared with Mkl1 KO, resulting in a statistically significant increase in 4N cells in DKO compared with Mkl1 KO mice. This suggests that MKL2 may play a role in promoting the second and subsequent endomitotic cycles as opposed to the first 2N to 4N endomitosis. There was a statistically significant decrease in mean ploidy of DKO CD41+ cells (n = 4) compared with WT (n = 4; P < .001) and Mkl1 KO (n = 4; P < .05) megakaryocytes. Consistent with the lack of a platelet phenotype, Mkl2 Pf4-cKO CD41+ BM cells did not show any difference from WT BM. The increased number of DKO BM megakaryocytes was confirmed by flow cytometry (Figure 4E); DKO marrow also had greater percentages of PreMegE and MkP versus WT (Figure 4F). As shown before,13,15 Mkl1 KO mice had a statistically significant (P < .05) increase over WT cells only in the MkP and total CD41+ populations. DKO megakaryocytes have a statistically significant increase in 4N megakaryocytes at the expense of ≥ 8N megakaryocytes compared with Mkl1 KO mice; this suggests that DKO mice have more impaired megakaryocyte maturation than Mkl1 KO mice.
Megakaryocytes from DKO mice have abnormal cytoplasmic ultrastructure
Ultrastructural analysis revealed morphologic abnormalities in DKO megakaryocytes (Figure 5A). Compared with WT, DKO megakaryocytes have cytoplasmic regions that are devoid of demarcation membrane and increased numbers of vacuoles, which normally appear during later stages of maturation (Figure 5Aiii,iv). In addition, the areas of microvesiculation that occur around the plasma membrane of WT megakaryocytes were present throughout the cytoplasm of DKO megakaryocytes in the process of proplatelet formation and may account for the heterogeneity in platelet shape, size, and granule content observed in these mice (Figure 5Aiii,iv). Analysis of Srf Pf4-cKO mice suggests that defects in the actin cytoskeleton are responsible for the abnormal megakaryocyte and platelet morphology.14 Measurement of the filamentous actin was determined by quantitative immunofluorescence microscopy of phalloidin (Figure 5B), which showed a significant decrease in actin organization in the DKO megakaryocytes, consistent with the hypothesis that the actin cytoskeleton is disordered.
Abnormal cytoskeleton in DKO megakaryocytes. (A) Representative thin-section electron micrographs of fetal liver–derived megakaryocytes from (i,ii) WT and (iii,iv) DKO embryos. (B) Phalloidin staining of fetal liver–derived megakaryocytes (top) and quantification of F-actin (bottom) show decreased polymerized actin in DKO (***P < .001; all error bars represent SEM).
Abnormal cytoskeleton in DKO megakaryocytes. (A) Representative thin-section electron micrographs of fetal liver–derived megakaryocytes from (i,ii) WT and (iii,iv) DKO embryos. (B) Phalloidin staining of fetal liver–derived megakaryocytes (top) and quantification of F-actin (bottom) show decreased polymerized actin in DKO (***P < .001; all error bars represent SEM).
RNA sequencing reveals SRF-independent functions for MRTFs in megakaryocyte differentiation
Investigation of the role of MKL2 in megakaryocytopoiesis suggests that MKL2 functions in the absence of MKL1 to promote megakaryocyte maturation, and that much of this effect is mediated via activation of SRF target genes. However, while the data indicate that many of the defects in DKO mice phenocopy those of SRF cKO mice, the platelet counts of DKO mice are more dramatically reduced. This may be because of SRF-independent functions of the MRTFs. To assess these novel functions, we sequenced the transcriptomes of WT, Mkl1 KO, Mkl2 Pf4-cKO, DKO, and Srf Pf4-cKO MkPs differentiated in vitro for 3 days with TPO. We confirmed that the population of differentiated megakaryocytes analyzed from each genotype consisted of a relatively pure (> 93%) CD41+/CD42+ cell population without myeloid contamination (data not shown).
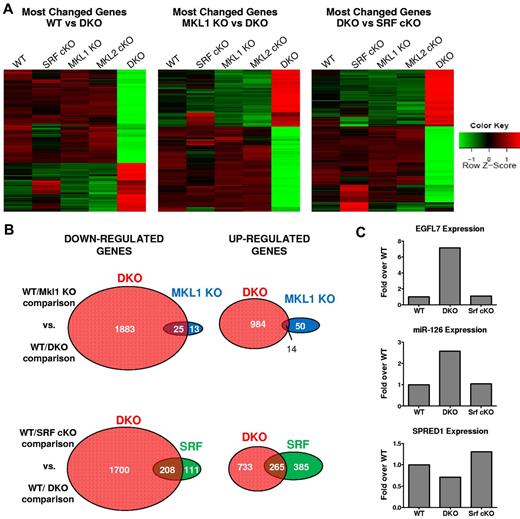
Analysis of the deep sequencing data revealed that DKO megakaryocytes had the most altered expression profile compared with the other 4 genotypes. A majority of the pairwise comparisons did not have many genes with greater than 2-fold differential gene expression (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). A total of 969 genes showed a > 2-fold difference between WT and Srf Pf4-cKO, while 2906 genes had a > 2-fold difference between WT and DKO megakaryocytes. Between Srf Pf4-cKO and DKO megakaryocytes, there were 3298 genes with a > 2-fold change in expression (Figure 6A-B, supplemental Figure 1). As expected, the DKO megakaryocytes significantly differed from Mkl1 KO megakaryocytes and consistent with the more extreme phenotype of the DKO mice, DKO megakaryocyte gene expression was more dissimilar to WT than any other genotype (Figure 6A, supplemental Figure 1). Although there were many genes that showed similar changes in gene expression in WT compared with DKO and Srf Pf4-cKO, most genes were DKO-specific (Figure 6B). The appearance of DKO-specific gene expression differences is unlikely because of incomplete removal of SRF in Srf Pf4-cKO, since there are also many genes that differ between Srf Pf4-cKO and WT, but not between DKO and WT (Figure 6B and data not shown). As expected, genes involved in cytoskeletal organization were significantly decreased in Mkl1 KO, DKO, and Srf Pf4-cKO megakaryocytes compared with WT (Table 2).
DKO and Srf Pf4-cKO megakaryocytes have distinct gene expression profiles. (A) Heat maps displaying the differential gene expression patterns of megakaryocytes from the indicated genotypes. Red color represents elevated expression while green represents decreased expression compared with the row mean. Genes displayed were selected based on fold changes of 2 or more and FDR adjusted P value < .05 between WT and DKO. (C) Venn diagrams showing genes with fold changes of 2 or more and FDR adjusted P value < .05 for the indicated comparisons. Representative qPCR expression of EGFL7, miR-126, and SPRED1 in megakaryocytes. Values are displayed as log2 fold change over WT.
DKO and Srf Pf4-cKO megakaryocytes have distinct gene expression profiles. (A) Heat maps displaying the differential gene expression patterns of megakaryocytes from the indicated genotypes. Red color represents elevated expression while green represents decreased expression compared with the row mean. Genes displayed were selected based on fold changes of 2 or more and FDR adjusted P value < .05 between WT and DKO. (C) Venn diagrams showing genes with fold changes of 2 or more and FDR adjusted P value < .05 for the indicated comparisons. Representative qPCR expression of EGFL7, miR-126, and SPRED1 in megakaryocytes. Values are displayed as log2 fold change over WT.
RNA sequencing cytoskeletal gene expression
| Gene . | FPKM . | ||||
|---|---|---|---|---|---|
| WT . | SRF cKO . | MKL1 KO . | MKL2 cKO . | DKO . | |
| Actb | 7795.17 | 2219.60* | 2696.76* | 5856.10 | 918.85* |
| Cfl1 | 1002.17 | 620.35 | 548.28 | 690.11 | 723.96 |
| Cnn2 | 247.85 | 102.00* | 42.33* | 220.86 | 41.44* |
| Flna | 839.82 | 304.74* | 309.33 | 583.87 | 56.52* |
| Myh9 | 999.37 | 631.94 | 1017.94 | 1311.00 | 107.44* |
| Pfn1 | 1935.96 | 918.24 | 1014.16 | 1359.71 | 1396.58 |
| Tpm2 | 1.04 | 1.02 | 0.54 | 1.03 | 4.08 |
| Tpm4 | 503.25 | 172.53* | 259.49 | 510.30 | 53.64* |
| Vcl | 201.99 | 65.07* | 99.46 | 171.20 | 12.10* |
| Gene . | FPKM . | ||||
|---|---|---|---|---|---|
| WT . | SRF cKO . | MKL1 KO . | MKL2 cKO . | DKO . | |
| Actb | 7795.17 | 2219.60* | 2696.76* | 5856.10 | 918.85* |
| Cfl1 | 1002.17 | 620.35 | 548.28 | 690.11 | 723.96 |
| Cnn2 | 247.85 | 102.00* | 42.33* | 220.86 | 41.44* |
| Flna | 839.82 | 304.74* | 309.33 | 583.87 | 56.52* |
| Myh9 | 999.37 | 631.94 | 1017.94 | 1311.00 | 107.44* |
| Pfn1 | 1935.96 | 918.24 | 1014.16 | 1359.71 | 1396.58 |
| Tpm2 | 1.04 | 1.02 | 0.54 | 1.03 | 4.08 |
| Tpm4 | 503.25 | 172.53* | 259.49 | 510.30 | 53.64* |
| Vcl | 201.99 | 65.07* | 99.46 | 171.20 | 12.10* |
RNA sequencing shows different expression levels of genes important in maintaining and remodeling the actin cytoskeleton. Reads represent fragments per kilobase per million and fold changes indicate log2 differences. Data are displayed in FPKM.
FPKM indicates fragments per kilobase of transcript per million; WT, wild type; SRF, serum response factor; KO, knockout; cKO, conditional knockout; and DKO, double knockout.
Differentially expressed genes reaching statistical significance (q < 0.05) compared to WT.
The differentially expressed genes open several interesting avenues of research that give insight into the cellular properties that drive the dramatic DKO platelet phenotype. We mined the RNA sequencing data for genes already defined by others as megakaryocyte-specific and genes highly expressed during megakaryocyte differentiation.23,24 Of the 56 genes curated by the Papoutsakis group as being megakaryocyte related by literature review, 24 were deregulated by loss of both MKL1 and MKL2 while only 11 of those same genes were affected by loss of SRF (Table 3). Of 58 transmembrane proteins enriched in megakaryocytes, 22 had altered gene expression in DKO megakaryocytes and 14 were deregulated in SRF Pf4-cKO megakaryocytes, while none of the 58 genes was significantly affected by loss of MKL1 alone (Table 4). Slc35d3, one of the genes that is significantly decreased uniquely in DKO cells, is essential for platelet dense granule biogenesis.25,26 Of the 35 genes that were up-regulated at least 16-fold compared with WT in both the Srf cKO and DKO (Table 5), 20 are strongly associated with defense response, particularly neutrophil and mast cell differentiation (for example, C/EBP epsilon). These data suggest that SRF, via activation by MRTFs, may act as a master switch to turn off myeloid differentiation programs during megakaryocyte commitment.
DKO megakaryocytes show deregulation of many megakaryocyte genes
| Gene . | FPKM . | ||||
|---|---|---|---|---|---|
| WT . | SRF cKO . | MKL1 KO . | MKL2 cKO . | DKO . | |
| Aurkb | 28.32 | 56.04 | 21.31 | 15.20 | 38.26 |
| Bcl2 | 2.05 | 1.58 | 2.42 | 2.13 | 0.91 |
| Bcl2l11 | 6.82 | 17.95* | 12.75 | 6.67 | 4.71 |
| Bcl2l1 | 177.03 | 75.50* | 141.81 | 170.54 | 63.91* |
| Cbfb | 32.56 | 35.92 | 30.95 | 23.69 | 10.49* |
| Ccl5 | 14.29 | 81.43* | 16.21 | 6.32 | 75.07* |
| Ccnd1 | 4.42 | 1.20 | 2.07 | 2.81 | 0.46 |
| Ccnd3 | 539.47 | 319.20 | 448.96 | 547.66 | 429.51 |
| Ccne1 | 5.36 | 7.25 | 4.45 | 2.90 | 9.88 |
| Ccne2 | 8.84 | 12.42 | 8.25 | 5.07 | 4.45 |
| Cd36 | 1.64 | 2.20 | 1.44 | 0.79 | 0.31 |
| Cd9 | 1630.72 | 852.78 | 1449.95 | 1627.59 | 619.83 |
| Cdkn1a | 346.36 | 255.60 | 328.10 | 292.71 | 228.05 |
| Cxcl12 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 |
| Cxcl5 | 52.68 | 41.74 | 51.20 | 87.20 | 10.92* |
| Cxcr4 | 11.60 | 104.87* | 10.61 | 9.35 | 41.40* |
| Ets1 | 6.50 | 18.58* | 9.49 | 8.98 | 3.80 |
| Etv6 | 83.30 | 73.60 | 85.93 | 109.23 | 13.31* |
| F2r | 538.79 | 403.48 | 510.62 | 754.55 | 73.00* |
| Fli1 | 92.75 | 62.78 | 74.83 | 107.55 | 16.70* |
| Gata1 | 136.72 | 61.85* | 100.83 | 131.26 | 93.43 |
| Gata2 | 32.63 | 29.09 | 31.35 | 22.08 | 47.30 |
| Gp1ba | 225.28 | 101.01* | 198.97 | 304.22 | 20.66* |
| Gp5 | 270.36 | 99.97* | 183.32 | 242.73 | 103.53 |
| Gp6 | 120.38 | 68.16 | 119.21 | 170.59 | 41.46* |
| Gp9 | 307.39 | 118.95* | 222.33 | 264.54 | 212.19 |
| Hsd3b1 | 0.19 | 0.04 | 0.13 | 0.08 | 0.01 |
| Hsd3b2 | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 |
| Itga2 | 8.72 | 4.07 | 10.33 | 21.05* | 0.08 |
| Itga2b | 2506.71 | 1231.66* | 2305.33 | 2301.65 | 1637.24 |
| Itga5 | 30.84 | 31.69 | 30.59 | 25.61 | 10.59 |
| Itgav | 16.04 | 17.03 | 18.47 | 20.23 | 14.37 |
| Itgb1 | 219.80 | 131.97 | 169.54 | 229.33 | 55.44* |
| Itgb3 | 1285.89 | 667.85 | 1130.56 | 1556.85 | 243.91 |
| Mafg | 16.36 | 9.91 | 16.54 | 19.66 | 1.24 |
| Mafk | 25.26 | 15.14 | 21.01 | 19.13 | 6.49* |
| Mcl1 | 112.29 | 129.28 | 127.35 | 88.38 | 57.66 |
| Mpl | 92.44 | 40.82* | 57.96 | 116.22 | 23.74* |
| Myb | 28.96 | 77.77* | 41.61 | 18.42 | 33.15 |
| Myh9 | 999.37 | 631.94 | 1017.94 | 1311.00 | 107.44* |
| Nfe2 | 620.83 | 357.27 | 532.17 | 550.66 | 390.86 |
| P2rx1 | 135.55 | 63.86* | 119.27 | 102.96 | 35.34* |
| P2ry1 | 38.38 | 21.97 | 38.53 | 45.93 | 5.06* |
| P2ry12 | 3.22 | 2.59 | 3.32 | 3.94 | 0.20 |
| Pf4 | 7444.13 | 4537.65 | 6066.01 | 6706.48 | 9408.82 |
| Ppbp | 5550.82 | 589.32* | 2275.74* | 8246.70 | 482.21* |
| Rab27b | 572.79 | 262.99* | 492.48 | 687.69 | 61.05* |
| Rabggta | 4.56 | 7.34 | 4.16 | 2.07 | 9.71 |
| Runx1 | 48.93 | 37.01 | 56.81 | 65.48 | 10.54* |
| Selp | 682.35 | 210.07* | 566.21 | 746.53 | 53.85* |
| Tal1 | 109.73 | 51.29* | 94.37 | 106.55 | 17.59* |
| Tbxas1 | 470.61 | 202.36* | 438.38 | 583.03 | 146.91* |
| Tnfrsf1a | 75.62 | 107.95 | 81.74 | 54.56 | 67.13 |
| Tubb1 | 119.49 | 85.87 | 107.73 | 183.81 | 12.97* |
| Vwf | 893.73 | 420.56 | 727.50 | 1220.57 | 313.62 |
| Zfpm1 | 121.21 | 58.22 | 72.54 | 115.08 | 18.29* |
| Gene . | FPKM . | ||||
|---|---|---|---|---|---|
| WT . | SRF cKO . | MKL1 KO . | MKL2 cKO . | DKO . | |
| Aurkb | 28.32 | 56.04 | 21.31 | 15.20 | 38.26 |
| Bcl2 | 2.05 | 1.58 | 2.42 | 2.13 | 0.91 |
| Bcl2l11 | 6.82 | 17.95* | 12.75 | 6.67 | 4.71 |
| Bcl2l1 | 177.03 | 75.50* | 141.81 | 170.54 | 63.91* |
| Cbfb | 32.56 | 35.92 | 30.95 | 23.69 | 10.49* |
| Ccl5 | 14.29 | 81.43* | 16.21 | 6.32 | 75.07* |
| Ccnd1 | 4.42 | 1.20 | 2.07 | 2.81 | 0.46 |
| Ccnd3 | 539.47 | 319.20 | 448.96 | 547.66 | 429.51 |
| Ccne1 | 5.36 | 7.25 | 4.45 | 2.90 | 9.88 |
| Ccne2 | 8.84 | 12.42 | 8.25 | 5.07 | 4.45 |
| Cd36 | 1.64 | 2.20 | 1.44 | 0.79 | 0.31 |
| Cd9 | 1630.72 | 852.78 | 1449.95 | 1627.59 | 619.83 |
| Cdkn1a | 346.36 | 255.60 | 328.10 | 292.71 | 228.05 |
| Cxcl12 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 |
| Cxcl5 | 52.68 | 41.74 | 51.20 | 87.20 | 10.92* |
| Cxcr4 | 11.60 | 104.87* | 10.61 | 9.35 | 41.40* |
| Ets1 | 6.50 | 18.58* | 9.49 | 8.98 | 3.80 |
| Etv6 | 83.30 | 73.60 | 85.93 | 109.23 | 13.31* |
| F2r | 538.79 | 403.48 | 510.62 | 754.55 | 73.00* |
| Fli1 | 92.75 | 62.78 | 74.83 | 107.55 | 16.70* |
| Gata1 | 136.72 | 61.85* | 100.83 | 131.26 | 93.43 |
| Gata2 | 32.63 | 29.09 | 31.35 | 22.08 | 47.30 |
| Gp1ba | 225.28 | 101.01* | 198.97 | 304.22 | 20.66* |
| Gp5 | 270.36 | 99.97* | 183.32 | 242.73 | 103.53 |
| Gp6 | 120.38 | 68.16 | 119.21 | 170.59 | 41.46* |
| Gp9 | 307.39 | 118.95* | 222.33 | 264.54 | 212.19 |
| Hsd3b1 | 0.19 | 0.04 | 0.13 | 0.08 | 0.01 |
| Hsd3b2 | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 |
| Itga2 | 8.72 | 4.07 | 10.33 | 21.05* | 0.08 |
| Itga2b | 2506.71 | 1231.66* | 2305.33 | 2301.65 | 1637.24 |
| Itga5 | 30.84 | 31.69 | 30.59 | 25.61 | 10.59 |
| Itgav | 16.04 | 17.03 | 18.47 | 20.23 | 14.37 |
| Itgb1 | 219.80 | 131.97 | 169.54 | 229.33 | 55.44* |
| Itgb3 | 1285.89 | 667.85 | 1130.56 | 1556.85 | 243.91 |
| Mafg | 16.36 | 9.91 | 16.54 | 19.66 | 1.24 |
| Mafk | 25.26 | 15.14 | 21.01 | 19.13 | 6.49* |
| Mcl1 | 112.29 | 129.28 | 127.35 | 88.38 | 57.66 |
| Mpl | 92.44 | 40.82* | 57.96 | 116.22 | 23.74* |
| Myb | 28.96 | 77.77* | 41.61 | 18.42 | 33.15 |
| Myh9 | 999.37 | 631.94 | 1017.94 | 1311.00 | 107.44* |
| Nfe2 | 620.83 | 357.27 | 532.17 | 550.66 | 390.86 |
| P2rx1 | 135.55 | 63.86* | 119.27 | 102.96 | 35.34* |
| P2ry1 | 38.38 | 21.97 | 38.53 | 45.93 | 5.06* |
| P2ry12 | 3.22 | 2.59 | 3.32 | 3.94 | 0.20 |
| Pf4 | 7444.13 | 4537.65 | 6066.01 | 6706.48 | 9408.82 |
| Ppbp | 5550.82 | 589.32* | 2275.74* | 8246.70 | 482.21* |
| Rab27b | 572.79 | 262.99* | 492.48 | 687.69 | 61.05* |
| Rabggta | 4.56 | 7.34 | 4.16 | 2.07 | 9.71 |
| Runx1 | 48.93 | 37.01 | 56.81 | 65.48 | 10.54* |
| Selp | 682.35 | 210.07* | 566.21 | 746.53 | 53.85* |
| Tal1 | 109.73 | 51.29* | 94.37 | 106.55 | 17.59* |
| Tbxas1 | 470.61 | 202.36* | 438.38 | 583.03 | 146.91* |
| Tnfrsf1a | 75.62 | 107.95 | 81.74 | 54.56 | 67.13 |
| Tubb1 | 119.49 | 85.87 | 107.73 | 183.81 | 12.97* |
| Vwf | 893.73 | 420.56 | 727.50 | 1220.57 | 313.62 |
| Zfpm1 | 121.21 | 58.22 | 72.54 | 115.08 | 18.29* |
Megakaryocyte curated genes based on a literature review as being important in megakaryocyte differentiation23 show differential gene expression between the different genotypes. Data are displayed in FPKM.
FPKM indicates fragments per kilobase of transcript per million; WT, wild type; SRF, serum response factor; KO, knockout; cKO, conditional knockout; and DKO, double knockout.
Differentially expressed genes reaching statistical significance (q < 0.05) compared to WT.
DKO and Srf Pf4-cKO megakaryocytes have differential megakaryocyte transmembrane gene expression
| Gene . | FPKM . | ||||
|---|---|---|---|---|---|
| WT . | SRF cKO . | MKL1 KO . | MKL2 cKO . | DKO . | |
| 1110008F13Rik | 168.39 | 108.66 | 124.53 | 118.02 | 162.05 |
| Alox5ap | 2324.05 | 1609.63 | 2435.91 | 2216.10 | 2811.82 |
| Atp5g3 | 385.29 | 244.15 | 250.03 | 242.72 | 508.98 |
| Bsg | 319.57 | 233.30 | 278.88 | 310.46 | 513.28 |
| Cd151 | 87.72 | 52.09 | 79.72 | 89.09 | 33.04* |
| Cd47 | 30.43 | 31.40 | 25.75 | 23.65 | 0.00 |
| Cd63 | 555.94 | 402.13 | 499.11 | 485.78 | 921.27 |
| Cd9 | 1630.72 | 852.78 | 1449.95 | 1627.59 | 619.83 |
| Cox4i1 | 521.67 | 401.89 | 414.34 | 408.12 | 1190.58 |
| Cox8a | 238.02 | 173.46 | 161.16 | 176.70 | 428.98 |
| Cyba | 155.52 | 323.36 | 134.54 | 87.42 | 765.77* |
| Dhcr24 | 148.52 | 76.21 | 124.12 | 133.68 | 32.72* |
| Dnajb4 | 42.37 | 25.26 | 36.92 | 47.77 | 11.62* |
| Esam | 126.13 | 68.79 | 80.62 | 146.31 | 91.92 |
| Eya2 | 31.29 | 14.01* | 41.15 | 24.88 | 10.51* |
| Fads2 | 207.86 | 130.48 | 172.31 | 254.37 | 50.26* |
| Fcer1g | 542.87 | 519.26 | 403.81 | 409.80 | 682.78 |
| Glipr1 | 245.40 | 205.21 | 238.57 | 161.53 | 227.67 |
| Gp1ba | 225.28 | 101.01* | 198.97 | 304.22 | 20.66* |
| Gp1bb | 68.88 | 31.31 | 47.20 | 51.64 | 73.57 |
| Gp49a | 812.14 | 447.61 | 810.08 | 724.34 | 192.64* |
| Gp5 | 270.36 | 99.97* | 183.32 | 242.73 | 103.53 |
| Gp9 | 307.39 | 118.95* | 222.33 | 264.54 | 212.19 |
| Gpr56 | 855.30 | 417.16 | 772.67 | 979.05 | 367.96 |
| H2-K1 | 602.78 | 843.12 | 527.17 | 490.93 | 1433.99* |
| Ifitm1 | 318.43 | 319.23 | 317.53 | 247.40 | 1536.24* |
| Ifitm2 | 263.50 | 278.33 | 236.67 | 250.93 | 501.76 |
| Ifitm3 | 580.84 | 578.90 | 410.03 | 463.05 | 1026.08 |
| Itga2b | 2506.71 | 1231.66* | 2305.33 | 2301.65 | 1637.24 |
| Itga6 | 665.79 | 277.35* | 625.44 | 736.21 | 50.74* |
| Itm2b | 691.15 | 965.94 | 734.92 | 600.35 | 739.78 |
| Laptm4a | 138.76 | 107.84 | 125.00 | 133.70 | 102.47 |
| Laptm5 | 2043.75 | 1786.38 | 2362.76 | 2407.72 | 512.81 |
| Lilrb4 | 1168.14 | 626.80 | 1169.76 | 1092.51 | 273.49* |
| Mcl1 | 112.29 | 129.28 | 127.35 | 88.38 | 57.66 |
| Mpl | 92.44 | 40.82* | 57.96 | 116.22 | 23.74* |
| Nrgn | 437.18 | 203.67 | 256.78 | 303.27 | 226.77 |
| P2rx1 | 135.55 | 63.86* | 119.27 | 102.96 | 35.34* |
| Pdzk1ip1 | 133.50 | 68.47 | 107.33 | 125.34 | 216.87 |
| Ptpro | 1.42 | 5.91 | 1.20 | 0.58 | 2.78 |
| Rpn1 | 108.33 | 90.37 | 90.52 | 80.59 | 87.08 |
| Scarb1 | 392.79 | 237.10 | 289.97 | 434.64 | 106.77* |
| Scd2 | 205.74 | 244.30 | 199.91 | 229.47 | 84.20 |
| Sec61a1 | 114.52 | 101.25 | 106.98 | 102.02 | 40.39 |
| Selp | 682.35 | 210.07* | 566.21 | 746.53 | 53.85* |
| Serinc3 | 523.01 | 476.27 | 552.93 | 434.22 | 177.98* |
| Serpinb10-ps | 176.75 | 79.74* | 150.23 | 142.55 | 46.67* |
| Serpinb2 | 1153.16 | 523.29* | 1393.18 | 1015.87 | 98.99* |
| Slc20a1 | 219.58 | 222.87 | 227.78 | 214.32 | 28.19* |
| Slc25a3 | 413.05 | 291.79 | 320.12 | 306.51 | 428.44 |
| Slc35d3 | 84.72 | 40.63 | 53.39 | 101.96 | 18.50* |
| Slc37a2 | 2.20 | 6.28 | 1.83 | 0.80 | 1.74 |
| Slc6a4 | 1717.84 | 542.43* | 1667.10 | 1172.70 | 71.01* |
| Ssr3 | 199.21 | 176.59 | 183.33 | 196.29 | 77.88 |
| Tmbim4 | 840.92 | 338.18* | 654.55 | 613.84 | 468.37 |
| Tmem40 | 363.85 | 145.47* | 267.51 | 294.62 | 264.04 |
| Tmem66 | 133.46 | 97.40 | 117.65 | 115.44 | 108.09 |
| Treml1 | 322.50 | 202.32 | 250.64 | 436.36 | 279.76 |
| Gene . | FPKM . | ||||
|---|---|---|---|---|---|
| WT . | SRF cKO . | MKL1 KO . | MKL2 cKO . | DKO . | |
| 1110008F13Rik | 168.39 | 108.66 | 124.53 | 118.02 | 162.05 |
| Alox5ap | 2324.05 | 1609.63 | 2435.91 | 2216.10 | 2811.82 |
| Atp5g3 | 385.29 | 244.15 | 250.03 | 242.72 | 508.98 |
| Bsg | 319.57 | 233.30 | 278.88 | 310.46 | 513.28 |
| Cd151 | 87.72 | 52.09 | 79.72 | 89.09 | 33.04* |
| Cd47 | 30.43 | 31.40 | 25.75 | 23.65 | 0.00 |
| Cd63 | 555.94 | 402.13 | 499.11 | 485.78 | 921.27 |
| Cd9 | 1630.72 | 852.78 | 1449.95 | 1627.59 | 619.83 |
| Cox4i1 | 521.67 | 401.89 | 414.34 | 408.12 | 1190.58 |
| Cox8a | 238.02 | 173.46 | 161.16 | 176.70 | 428.98 |
| Cyba | 155.52 | 323.36 | 134.54 | 87.42 | 765.77* |
| Dhcr24 | 148.52 | 76.21 | 124.12 | 133.68 | 32.72* |
| Dnajb4 | 42.37 | 25.26 | 36.92 | 47.77 | 11.62* |
| Esam | 126.13 | 68.79 | 80.62 | 146.31 | 91.92 |
| Eya2 | 31.29 | 14.01* | 41.15 | 24.88 | 10.51* |
| Fads2 | 207.86 | 130.48 | 172.31 | 254.37 | 50.26* |
| Fcer1g | 542.87 | 519.26 | 403.81 | 409.80 | 682.78 |
| Glipr1 | 245.40 | 205.21 | 238.57 | 161.53 | 227.67 |
| Gp1ba | 225.28 | 101.01* | 198.97 | 304.22 | 20.66* |
| Gp1bb | 68.88 | 31.31 | 47.20 | 51.64 | 73.57 |
| Gp49a | 812.14 | 447.61 | 810.08 | 724.34 | 192.64* |
| Gp5 | 270.36 | 99.97* | 183.32 | 242.73 | 103.53 |
| Gp9 | 307.39 | 118.95* | 222.33 | 264.54 | 212.19 |
| Gpr56 | 855.30 | 417.16 | 772.67 | 979.05 | 367.96 |
| H2-K1 | 602.78 | 843.12 | 527.17 | 490.93 | 1433.99* |
| Ifitm1 | 318.43 | 319.23 | 317.53 | 247.40 | 1536.24* |
| Ifitm2 | 263.50 | 278.33 | 236.67 | 250.93 | 501.76 |
| Ifitm3 | 580.84 | 578.90 | 410.03 | 463.05 | 1026.08 |
| Itga2b | 2506.71 | 1231.66* | 2305.33 | 2301.65 | 1637.24 |
| Itga6 | 665.79 | 277.35* | 625.44 | 736.21 | 50.74* |
| Itm2b | 691.15 | 965.94 | 734.92 | 600.35 | 739.78 |
| Laptm4a | 138.76 | 107.84 | 125.00 | 133.70 | 102.47 |
| Laptm5 | 2043.75 | 1786.38 | 2362.76 | 2407.72 | 512.81 |
| Lilrb4 | 1168.14 | 626.80 | 1169.76 | 1092.51 | 273.49* |
| Mcl1 | 112.29 | 129.28 | 127.35 | 88.38 | 57.66 |
| Mpl | 92.44 | 40.82* | 57.96 | 116.22 | 23.74* |
| Nrgn | 437.18 | 203.67 | 256.78 | 303.27 | 226.77 |
| P2rx1 | 135.55 | 63.86* | 119.27 | 102.96 | 35.34* |
| Pdzk1ip1 | 133.50 | 68.47 | 107.33 | 125.34 | 216.87 |
| Ptpro | 1.42 | 5.91 | 1.20 | 0.58 | 2.78 |
| Rpn1 | 108.33 | 90.37 | 90.52 | 80.59 | 87.08 |
| Scarb1 | 392.79 | 237.10 | 289.97 | 434.64 | 106.77* |
| Scd2 | 205.74 | 244.30 | 199.91 | 229.47 | 84.20 |
| Sec61a1 | 114.52 | 101.25 | 106.98 | 102.02 | 40.39 |
| Selp | 682.35 | 210.07* | 566.21 | 746.53 | 53.85* |
| Serinc3 | 523.01 | 476.27 | 552.93 | 434.22 | 177.98* |
| Serpinb10-ps | 176.75 | 79.74* | 150.23 | 142.55 | 46.67* |
| Serpinb2 | 1153.16 | 523.29* | 1393.18 | 1015.87 | 98.99* |
| Slc20a1 | 219.58 | 222.87 | 227.78 | 214.32 | 28.19* |
| Slc25a3 | 413.05 | 291.79 | 320.12 | 306.51 | 428.44 |
| Slc35d3 | 84.72 | 40.63 | 53.39 | 101.96 | 18.50* |
| Slc37a2 | 2.20 | 6.28 | 1.83 | 0.80 | 1.74 |
| Slc6a4 | 1717.84 | 542.43* | 1667.10 | 1172.70 | 71.01* |
| Ssr3 | 199.21 | 176.59 | 183.33 | 196.29 | 77.88 |
| Tmbim4 | 840.92 | 338.18* | 654.55 | 613.84 | 468.37 |
| Tmem40 | 363.85 | 145.47* | 267.51 | 294.62 | 264.04 |
| Tmem66 | 133.46 | 97.40 | 117.65 | 115.44 | 108.09 |
| Treml1 | 322.50 | 202.32 | 250.64 | 436.36 | 279.76 |
Data obtained from our RNA sequencing was compared to a list of the transmembrane proteins expressed specifically in megakaryocytes or up-regulated in megakaryocyte differentiation.24 Data are displayed in FPKM.
FPKM indicates fragments per kilobase of transcript per million; WT, wild type; SRF, serum response factor; KO, knockout; cKO, conditional knockout; and DKO, double knockout.
Differentially expressed genes reaching statistical significance (q < 0.05) compared to WT.
Top genes increased in both DKO and Srf Pf4-cKO megakaryocytes
| Gene . | FPKM . | ||||
|---|---|---|---|---|---|
| WT . | SRF cKO . | MKL1 KO . | MKL2 cKO . | DKO . | |
| Camp | 3.55 | 217.84 | 2.54 | 0.68 | 365.42 |
| Cd177 | 0.62 | 25.74 | 0.49 | 0.29 | 19.06 |
| Cebpe | 0.99 | 38.18 | 1.03 | 0.48 | 78.54 |
| Cldn15 | 2.83 | 50.78 | 3.05 | 1.93 | 51.11 |
| Cma1 | 0.26 | 15.85 | 0.25 | 0.14 | 24.63 |
| Cpa3 | 2.95 | 46.38 | 4.18 | 2.93 | 53.55 |
| Ctsg | 80.04 | 1110.46 | 77.54 | 42.09 | 1436.34 |
| Dmkn | 4.20 | 30.77 | 4.87 | 2.36 | 73.51 |
| Elane | 126.51 | 2657.87 | 129.82 | 76.19 | 4523.18 |
| Epx | 1.39 | 16.91 | 0.79 | 1.00 | 21.83 |
| Fcnb | 2.37 | 196.80 | 4.08 | 1.98 | 107.95 |
| G0s2 | 0.41 | 10.25 | 0.43 | 0.20 | 15.04 |
| Gfi1 | 1.78 | 70.69 | 2.11 | 1.07 | 39.86 |
| Gstm1 | 10.90 | 202.47 | 12.81 | 5.97 | 231.21 |
| Lcn2 | 46.34 | 447.48 | 55.93 | 24.45 | 774.36 |
| Ltf | 1.64 | 65.56 | 1.09 | 0.65 | 35.35 |
| Mapk13 | 0.66 | 12.56 | 0.54 | 0.29 | 18.51 |
| Mcpt8 | 7.46 | 141.38 | 10.37 | 6.24 | 362.91 |
| Mgst2 | 5.72 | 104.82 | 3.61 | 2.69 | 158.53 |
| Mogat2 | 0.48 | 33.16 | 0.44 | 0.46 | 19.44 |
| Ms4a3 | 33.51 | 348.80 | 38.93 | 19.18 | 483.45 |
| Ngp | 9.93 | 764.69 | 5.76 | 2.56 | 834.41 |
| Oscp1 | 0.74 | 10.77 | 0.75 | 0.74 | 13.66 |
| Pglyrp1 | 0.86 | 79.38 | 1.25 | 0.59 | 204.23 |
| Prg2 | 19.58 | 220.00 | 19.20 | 14.11 | 858.80 |
| Prg3 | 2.13 | 17.73 | 1.91 | 1.44 | 71.50 |
| Prss34 | 3.89 | 81.31 | 5.31 | 2.95 | 273.43 |
| Prss57 | 0.95 | 36.18 | 1.00 | 0.60 | 76.53 |
| Prtn3 | 123.47 | 1195.12 | 105.27 | 57.77 | 2720.61 |
| Ramp1 | 1.50 | 15.73 | 1.72 | 0.75 | 22.26 |
| S100a8 | 237.63 | 8015.96 | 226.82 | 68.84 | 9008.47 |
| S100a9 | 137.93 | 7049.68 | 144.50 | 44.80 | 9372.56 |
| Saa3 | 606.97 | 3825.61 | 628.93 | 244.01 | 9136.90 |
| Slpi | 30.75 | 244.71 | 36.52 | 17.42 | 663.24 |
| Tst | 1.56 | 15.88 | 1.14 | 1.26 | 35.82 |
| Gene . | FPKM . | ||||
|---|---|---|---|---|---|
| WT . | SRF cKO . | MKL1 KO . | MKL2 cKO . | DKO . | |
| Camp | 3.55 | 217.84 | 2.54 | 0.68 | 365.42 |
| Cd177 | 0.62 | 25.74 | 0.49 | 0.29 | 19.06 |
| Cebpe | 0.99 | 38.18 | 1.03 | 0.48 | 78.54 |
| Cldn15 | 2.83 | 50.78 | 3.05 | 1.93 | 51.11 |
| Cma1 | 0.26 | 15.85 | 0.25 | 0.14 | 24.63 |
| Cpa3 | 2.95 | 46.38 | 4.18 | 2.93 | 53.55 |
| Ctsg | 80.04 | 1110.46 | 77.54 | 42.09 | 1436.34 |
| Dmkn | 4.20 | 30.77 | 4.87 | 2.36 | 73.51 |
| Elane | 126.51 | 2657.87 | 129.82 | 76.19 | 4523.18 |
| Epx | 1.39 | 16.91 | 0.79 | 1.00 | 21.83 |
| Fcnb | 2.37 | 196.80 | 4.08 | 1.98 | 107.95 |
| G0s2 | 0.41 | 10.25 | 0.43 | 0.20 | 15.04 |
| Gfi1 | 1.78 | 70.69 | 2.11 | 1.07 | 39.86 |
| Gstm1 | 10.90 | 202.47 | 12.81 | 5.97 | 231.21 |
| Lcn2 | 46.34 | 447.48 | 55.93 | 24.45 | 774.36 |
| Ltf | 1.64 | 65.56 | 1.09 | 0.65 | 35.35 |
| Mapk13 | 0.66 | 12.56 | 0.54 | 0.29 | 18.51 |
| Mcpt8 | 7.46 | 141.38 | 10.37 | 6.24 | 362.91 |
| Mgst2 | 5.72 | 104.82 | 3.61 | 2.69 | 158.53 |
| Mogat2 | 0.48 | 33.16 | 0.44 | 0.46 | 19.44 |
| Ms4a3 | 33.51 | 348.80 | 38.93 | 19.18 | 483.45 |
| Ngp | 9.93 | 764.69 | 5.76 | 2.56 | 834.41 |
| Oscp1 | 0.74 | 10.77 | 0.75 | 0.74 | 13.66 |
| Pglyrp1 | 0.86 | 79.38 | 1.25 | 0.59 | 204.23 |
| Prg2 | 19.58 | 220.00 | 19.20 | 14.11 | 858.80 |
| Prg3 | 2.13 | 17.73 | 1.91 | 1.44 | 71.50 |
| Prss34 | 3.89 | 81.31 | 5.31 | 2.95 | 273.43 |
| Prss57 | 0.95 | 36.18 | 1.00 | 0.60 | 76.53 |
| Prtn3 | 123.47 | 1195.12 | 105.27 | 57.77 | 2720.61 |
| Ramp1 | 1.50 | 15.73 | 1.72 | 0.75 | 22.26 |
| S100a8 | 237.63 | 8015.96 | 226.82 | 68.84 | 9008.47 |
| S100a9 | 137.93 | 7049.68 | 144.50 | 44.80 | 9372.56 |
| Saa3 | 606.97 | 3825.61 | 628.93 | 244.01 | 9136.90 |
| Slpi | 30.75 | 244.71 | 36.52 | 17.42 | 663.24 |
| Tst | 1.56 | 15.88 | 1.14 | 1.26 | 35.82 |
A list of genes more than 16-fold increased in DKO and Srf Pf4-cKO megakaryocytes compared to WT megakaryocytes. Data are displayed in FPKM.
FPKM indicates fragments per kilobase of transcript per million; WT, wild type; SRF, serum response factor; KO, knockout; cKO, conditional knockout; and DKO, double knockout.
Epidermal growth factor-like domain 7 (Egfl7) was the most significantly overexpressed gene in DKO compared with WT, Mkl1 KO, Mkl2 Pf4-cKO, and Srf Pf4-cKO megakaryocytes. Its expression was up-regulated 10-fold in DKO megakaryocytes by RNA sequencing analysis and greater than 6-fold by quantitative PCR analysis (Figure 6C and data not shown). Egfl7 protein is expressed and secreted by endothelial cells, and Egfl7 knockdown in primary human endothelial cells results in decreased proliferation and migration.27 Within Egfl7's seventh intron is miR-126. Consistent with the Egfl7 data, expression of miR-126 as determined by quantitative PCR is also significantly increased in DKO megakaryocytes (Figure 6C). Gene set enrichment analysis28,29 confirmed the decrease in expression of miR-126 target genes in DKO megakaryocytes compared with WT megakaryocytes (supplemental Figure 2). RNA sequencing and qPCR of DKO megakaryocytes showed decreased expression of Spred1, a published target of miR-12630 (Figure 6C and data not shown). Therefore, the more extreme megakaryocyte and platelet phenotype in the DKO mice compared with Srf Pf4-cKO mice is independent of the SRF cotranscriptional activities of MKL1 and MKL2.
Discussion
Megakaryocytes lacking expression of MKL1 and MKL2 have both defective megakaryocytopoiesis and thrombopoiesis. Mkl1 KO mice with megakaryocyte-specific loss of MKL2 have increased megakaryocyte progenitors and immature megakaryocytes in the BM. DKO mice have macrothrombocytopenia and increased bleeding times as a result of ineffective megakaryocytopoiesis and defects in platelet formation and activation. Many, but not all, of the differences between the Mkl1 KO and the SRF Pf4-cKO can be explained by the presence of MKL2.
This study is the first to describe a role for MKL2 in hematopoiesis. Although MKL2 was known to function in the brain and smooth muscle cells,8,16,31 investigators had dismissed MKL2 as a mediator of megakaryocytopoiesis because expression in human megakaryocytes is low.32 While our mouse studies also show that MKL2 is not as highly expressed as MKL1, megakaryocyte-specific deletion of MKL2 in the absence of MKL1 indicates that MKL2 plays a role, albeit redundant, in megakaryocytopoiesis. SRF target genes that are differentially expressed in the absence of SRF, MKL1, or MKL2 have been determined by this study and others.14,15 Future studies will be focused on determining how these differentially expressed target genes cause the differences in phenotypes between the DKO and Srf Pf4-cKO megakaryocytes and platelets. To further determine the mechanisms by which MKL1 and MKL2 act to promote megakaryocytopoiesis, it will be essential to know where SRF, MKL1, and MKL2 are bound on the chromatin during megakaryocyte differentiation. Furthermore, it will be important to determine whether the ability of MKL2 to compensate for MKL1 is because of MKL2 occupation of the genomic sites left vacant by the MKL1 deficiency in an Mkl1 KO mouse.
Comparison of ploidy between Mkl1 KO and DKO BM megakaryocytes indicates that DKO mice have a greater proportion of megakaryocytes in the 4N stage and a lower percentage with ≥ 8N DNA content. Recently, a relationship between MKL1 and guanine nucleotide exchange factors (GEFs) that control the final stages of cytokinesis was shown.33 GEF-H1 is a Rho-activating protein that localizes to the contractile ring during normal mitosis. In normal differentiation, GEF-H1 expression decreases before the first megakaryocyte endomitotic cycle and then increases as maturation continues. Not only do Mkl1 KO megakaryocytes have increased levels of GEF-H1, but Mkl1 KO megakaryocytes also do not down regulate GEF-H1 expression at any time during megakaryocyte maturation. SRF binds at the GEF-H1 promoter in hematopoietic cells, indicating a direct link between MKL1 and GEF-H1 regulation.34
ECT2, another GEF, also plays a role in megakaryocyte endomitosis. ECT2 concentrates in the midzone during cleavage furrow formation and is also required for the final stages of cytokinesis. Disruption of ECT2 function leads to polyploidy in HeLa cells.35 ECT2 is down-regulated in normal megakaryocytes at the second endomitotic cycle (4N to 8N) and remains low as maturation proceeds. RNA sequencing of WT, Mkl1 KO, Mkl2 Pf4-cKO, DKO, and Srf Pf4-cKO mature megakaryocytes revealed higher expression of ECT2 in DKO and Srf Pf4-cKO samples. There was no significant difference in GEF-H1 expression between WT and Mkl1 KO mature megakaryocytes in these data sequencing data, which is in agreement with the initial report for megakaryocyte progenitors cultured for 3 days in vitro. It may be that MKL1 and MKL2 differentially affect the down-regulation of GEFs, which needs to occur to promote endomitosis. Future studies may be performed on megakaryocytes from Mkl1 KO and DKO mice to assess the levels of critical GEFs that need to be down-regulated for the different stages of polyploidization and maturation.
Analysis of differentially regulated genes and the abnormal morphology of DKO megakaryocytes suggest that MKL1 and MKL2, through their interactions with SRF, control the complex architectural rearrangements that occur to allow proplatelet formation and, ultimately, platelet release. One of the genes that is decreased in the DKO, the Srf Pf4-cKO, and the Srf Mx1-cKO is Filamin A (Flna).14,15 The Flna Pf4-cKO was recently reported to have macrothrombocytopenia.36 This phenotype is consistent with the phenotype of DKO platelets and the Srf cKO platelets. However, the Flna Pf4-cKO mice have structurally competent megakaryocytes, indicating the more pleiotropic nature of the SRF/MKL deficiency. Future individual analyses of the major differentially expressed genes reported in the Srf Pf4-cKO microarray should allow a more comprehensive understanding of the many ways in which the MRTF/SRF pathway contributes to megakaryocytopoiesis and ultimately to thrombopoiesis.
One reason why DKO mice have fewer platelets than Srf Pf4-cKO mice may be the presence of residual SRF protein in megakaryocytes because the Pf4 promoter is not activated until after megakaryocyte commitment occurs. Although a 90% decrease in SRF RNA was confirmed, assessment of SRF protein levels was not done.14 The Srf Mx-1 Cre mice, in which SRF is knocked out in all hematopoietic cells after administration of Poly(I):Poly(C), have platelet counts more similar to our DKO mice, supporting the residual protein hypothesis.15 Another possibility is that the differences in phenotype between the DKO and Srf Pf4-cKO mice may be attributable to SRF-independent functions of the MRTFs. It has recently been shown that MKL1 can activate genes independently of its ability to bind SRF.37
RNA quantification via deep sequencing revealed potential genes that could contribute to the phenotypic differences seen in DKO and Srf Pf4-cKO mice. Egfl7 and miR-126 are intriguing candidates for further study. Egfl7 is highly expressed in endothelial cells. The promoter region critical for its activation contains 2 Ets-binding sites. Both Ets-1 and Ets-2 transactivate the Egfl7 promoter in luciferase assays.38 These proteins are members of the same superfamily as the ternary complex factors (TCFs), which compete with MRTFs for SRF binding.39
Expression of Egfl7 correlates with miR-126 expression whose function in megakaryocytopoiesis is unclear. Down-regulation of Spred1 by the increase in miR-126 expression drives proliferation and activation of mast cells,40 functionally linking our observations of increased miR-126 expression with an increase in mast cell–related gene expression in the DKO megakaryocytes. Reports from zebrafish categorize c-Myb as a direct target of miR-126. Morpholino knockdown of miR-126 results in increased c-Myb expression, which promotes red cell production at the expense of thrombocytes.41 Overexpression of miR-126 in human embryonic stem-cell derived CD34+ cells leads to a decrease in erythroid colony formation; megakaryocyte potential was not assessed by this report.42 However, expression of miR-126 decreases with megakaryocyte differentiation from adult CD34+ cells.43 Lastly, several groups have reported the aberrant expression of miR-126 in acute myeloid leukemia-initiating cells.43-45 These reports suggest that deregulation of miR-126 and other miRs (eg, miR-155) may be the additional hits that drive leukemic progression. This suggests an intriguing hypothesis in the case of t(1;22) AMKL; the fusion protein OTT-MKL1 may disrupt the balance of TCFs and MRTFs at the Egfl7 promoter similar to the DKO mice. The result is the production of hyperproliferative cells that have impaired megakaryocyte differentiation because of lack of normal MKL1 expression. This is also supported by competitive repopulation experiments in which overexpression of miR-126 provides an engraftment advantage.46 These findings reveal a connection between the MRTFs, Ets domain proteins, and miR-126 highlighting a pathway that may give insight into the pathogenesis of AMKL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Eric Olson for the Mkl2 floxed mice, Dr Stephen Morris for the Mkl1 KO mice, and Stephanie Donaldson for excellent mouse husbandry. The authors also thank Dr Emanuela Bruscia, Alexandra Teixeira, Dr Betty Lawton, and Dr Dennis Jones for thoughtful insights and careful editing of this manuscript.
This work was supported by National Institutes of Health (NIH) grant F31 HL 094118 (to E.C.S.), and by NIH grants DK086267, DK072442 (Yale Center of Excellence in Molecular Hematology), and the Connecticut Stem Cell Fund (to D.S.K.), and HL106184 (to P.G.).
National Institutes of Health
Authorship
Contribution: E.C.S. designed and performed experiments and wrote the manuscript; J.N.T., M.T.D., S.L., Y.G., and S.A.M. performed experiments and provided technical expertise; V.P.S. performed bioinformatics analysis; S.H., P.G., and J.E.I. contributed scientific knowledge; and D.S.K. provided mentorship and intellectual input, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Diane S. Krause, Department of Laboratory Medicine, Yale University School of Medicine, PO Box 208073, 333 Cedar St, New Haven, CT 06520-8073; e-mail: diane.krause@yale.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal