Abstract
Invariant natural killer T (iNKT) cells can experimentally dissociate GVL from graft-versus-host-disease (GVHD). Their role in human conventional allogeneic hematopoietic stem cell transplantation (HSCT) is unknown. Here, we analyzed the post-HSCT recovery of iNKT cells in 71 adult allografted patients. Results were compared with conventional T- and NK-cell recovery and correlated to the occurrence of GVHD, relapse, and survival. We observed that posttransplantation iNKT cells, likely of donor origin, recovered independently of T and NK cells in the first 90 days after HSCT and reached greater levels in recipient younger than 45 years (P = .003) and after a reduced-intensity conditioning regimen (P = .03). Low posttransplantation iNKT/T ratios (ie, < 10−3) were an independent factor associated with the occurrence of acute GVHD (aGVHD; P = .001). Inversely, reaching iNKT/T ratios > 10−3 before day 90 was associated with reduced nonrelapse mortality (P = .009) without increased risk of relapse and appeared as an independent predictive factor of an improved overall survival (P = .028). Furthermore, an iNKT/T ratio on day 15 > 0.58 × 10−3 was associated with a 94% risk reduction of aGVHD. These findings provide a proof of concept that early postallogeneic HSCT iNKT cell recovery can predict the occurrence of aGVHD and an improved overall survival.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) remains the only curative treatment for many hematologic malignancies.1 However, the development of an antileukemic response (GVL effect) often is hampered by the concomitant occurrence of a life-threatening graft-versus-host disease (GVHD).2 The treatment of GVHD requires intensification of immunosuppressive therapy that may be responsible for an impaired immune reconstitution, leading to an increase risk of life-threatening infections and alteration of the GVL effect. The main challenge in allogeneic HSCT is to define strategies that could predict and prevent GVHD without impairing the GVL effect and the posttransplantation immune reconstitution.
Several pretransplantation characteristics have been well established as predictive factors of acute GVHD (aGVHD)3 but are without significant impact in clinical practice. The determination of posttransplantation predictive factors should be of greater help in the development of new preventive or curative therapy of aGVHD.
Although not clearly established, GVL and GVHD share common pathophysiologic pathways. However, there is some clinical and biologic evidence that they can occur separately as a consequence of distinct alloreactive T-cell clone activation.4,5 In mouse models, numerous approaches inhibiting some of the pathways implicated in the pathophysiology of GVHD have been shown to prevent GVHD without impairing the GVL effect, but so far none has been successfully applied in the clinical setting. Among these strategies, the use of immunoregulatory T cells, suppressive subsets of naturally occurring T cells, recently has generated a lot of interest.6
In patients, a reduction of peripheral CD4+CD25+FoxP3+ CD127low regulatory T lymphocytes (Tregs), and altered Treg functions have been reported in patients who develop acute GVHD.7,8 However, the feasibility and efficacy of controlling acute GVHD by administering such Tregs remains to be demonstrated,9,10 and concern exists as to their antitumor suppressive activity.11 A defect of Tregs also has been described in patients who develop chronic GVHD.12,13 In this setting, an in vivo expansion of regulatory T cells by the administration of low doses of IL-2 recently has been shown to be associated with a clinical improvement of GVHD symptoms.14
Another T-cell population endowed with regulatory functions, namely invariant natural killer T (iNKT) cells, has elicited much interest in recent years because of its implication in several immune responses, including immune tolerance and tumor surveillance.15-17 iNKT cells express a highly restricted T-cell receptor (TCR) repertoire, composed of a single invariant chain (Vα14Jα18 in mice and a Vα24Jα18 in humans).18 In contrast to conventional T cells, the invariant TCR endows iNKT cells with the unique property of responding to glycolipids presented by the nonpolymorphic class I–like molecule CD1d.15,19 Once activated, iNKT cells promptly produce large amounts of cytokines,19-22 enabling them to regulate the activation, function, and proliferation of multiple immune effectors.15,20-24
In mouse models of GVHD, iNKT cells can inhibit or exacerbate GVHD, depending on models and type of activation, and preserve the GVL effect.25-31 In humans, reconstitution of iNKT cells after allogeneic HSCT has been poorly explored. Here, we report that early (day 15 after transplantation) iNKT/T-cell ratio can predict the occurrence of aGVHD and that enhanced posttransplantation iNKT reconstitution is a predictive factor of an improved overall survival (OS) because of a risk reduction of aGVHD without impairment of the GVL effect. In addition to the interest in monitoring GVHD, these data suggest that iNKT cells might be a new target for the prevention and/or treatment of GVHD.
Methods
Patients
Between February 2006 and April 2011, 71 patients who underwent an allogeneic HSCT in 2 transplantation institutions were prospectively entered in this study after providing their informed consent. The study was conducted according to the procedures of the Declaration of Helsinki and to local ethic committee rules.
Flow cytometry analysis
Blood samples were obtained before allogeneic HSCT, on the day of transplantation, and every 2 weeks thereafter for 3 months. All analyses were performed on freshly isolated peripheral blood mononuclear cells (PBMCs) from 10 to 20 mL of blood by density-gradient centrifugation (Ficoll-Paque PLUS; GE Healthcare) within the 12 hours after blood was drawn. Cell-surface staining was performed in PBS buffer containing 2% FCS and 0.01% NaN3 on ice. Cells were first stained with PBS57-loaded or empty-CD1d-tetramers (National Institutes of Health Tetramer Core Facility), then with the following directly conjugated monoclonal antibodies (eBioscience): anti-CD3, anti-CD4, anti-CD8, and anti-CD56. Data were acquired on a FACSCanto II flow cytometer (BD Biosciences) with the use of FACSDiva Version 6.1.3 software (BD Biosciences) and were analyzed with the FlowJo Version 7.6.5 software (TreeStar). Lymphocyte subpopulations were analyzed within the lymphocyte gate on forward and side-scatter plots. Results were expressed in absolute numbers or in terms of iNKT/103 T-lymphocyte ratios. Because iNKT cells represent a rare population of T lymphocytes, data were considered as reliable when a minimum of 1 × 106 PBMCs were stained, and at least 5 × 104 CD3+ T cells with a minimum of 20 iNKT CD3+CD1dTetramer+ cells were acquired to provide the most accurate iNKT/T ratios, as represented in Figure 1A.
Chimerism analysis
PBMCs were cultured at a density of 106 cells per mL in RPMI 1640 medium containing antibiotics, 10% FBS, 200mM glutamine, and 10mM HEPES (all from Life Technologies, Invitrogen) in the presence of α-galactosylceramide (100 ng/mL; Alexis Biochem–Enzo Life Sciences). RhIL-2 (50 ng/mL; Immunotools) was added to the culture 24 hours later. After 2 weeks, cells were stained with anti-CD3, anti-CD4, and CD1d-tetramers and sorted on a FACSAria (BD Biosciences) as CD1d-Tetramer–positive iNKT cells and CD4+CD1d− Tetramer–negative conventional T cells. Genomic DNA was extracted from both subtype of sorted cells with the Quick-Gene 610L DNA extraction system (Fujifilm LifeSciences) and subjected to real-time single-nucleotide polymorphism PCR for quantitative detection of chimerism.
Functional studies of iNKT cells
Cytokine secretion was assessed after stimulation of expanded iNKT cells with phorbol myristate acetate (25 ng/mL) plus ionomycin (1 μg/mL; both from Sigma-Aldrich) for 48 hours. IL-4 and IFN-γ were measured in culture supernatants by DuoSet ELISA (R&D Systems). Data were analyzed with the SOFTMAXPRO Version 5.2 software.
Statistical analyses
Statistical analyses were performed with SAS Version 9.2 for PC (SAS Institute), SPSS Version 20.0 for Macintosh, R Version 2.7.0 statistical software,32 and GraphPad Prism Version 5.01 for Windows.
The generalized estimating equation (GEE) approach was used to assess the association of repeated measurements (on days 15, 30, 60, and 90 after HSCT) of dichotomized iNKT/T ratio (< or ≥ 10−3) and explanatory parameters (Table 2). Odds ratios were calculated to reflect the potential associations between determinants and outcomes. Interaction terms with time were incorporated into all models for baseline parameters, and none was found significant.
OS was measured from the date of HSCT until date of death from any cause or date of last contact. Nonrelapse mortality (NRM) was defined as death occurring in continuous complete remission (CR).
Univariate and multivariate Cox proportional hazards models with iNKT/T ratio considered as a time-dependent variable were used to assess the predictive value of iNKT cell reconstitution on the development of aGVHD or CMV reactivation as well as the predictive value of aGVHD development on the risk of CMV reactivation. Of note, despite a relatively small number of patients (n = 71) who underwent allografts, all multivariate models used in the study converged. It should be acknowledged that limited size of patient subgroups and consequent restricted statistical power could have precluded reaching significance for some comparisons.
To assess the prognostic value of iNKT cell recovery on NRM, relapse incidence, and OS, all survival analyses were conducted as landmark analyses and performed with a landmark time set at day 90 after HSCT. To calculate the cumulative incidence of relapse, NRM was considered as a competitive risk, and the statistical significance of iNKT/T ratio was assessed by use of the Gray test.33 The OS curve was established with the Kaplan-Meier method and statistical differences in survival distributions according to baseline parameters and iNKT/T ratio were assessed with the logrank test. The Cox proportional hazard model was used for multivariate analyses of OS. Of note, cumulative incidence also was used to estimate the probability of each subtype of GVHD (acute and chronic) as well as CMV reactivation; we treated death that was unrelated to the event as a competing risk.
For all multivariate analyses (GEE, time-independent or time-dependent Cox models), stepwise regression consisting of alternating forward and backward elimination steps was used to find the most parsimonious set of covariates with statistical significance. The level for entry into the model was set at 0.2 with a stay level of 0.05.
To assess not only the association but also the ability of iNKT/T ratio to predict the occurrence of aGVHD, the value of the ratio at day 15 after transplantation and actual development of aGVHD were subjected to receiver operating characteristic (ROC) curve analysis.34 For each cut-off point, the resulting sensitivity and specificity were indicated as a point on a graph. The bootstrap method was used to calculate the area under the curve (AUC) of the ROC curve and 95% confidence intervals (CIs). Because cut-points are needed in routine practice to provide guidelines for medical decision-making, an optimal cut-off point was selected on the basis of the iNKT/T ratio that provided the best balance of sensitivity and specificity (ie, which at the same time minimized the Euclidean distance between the corresponding point on the ROC curve and the perfect predictor, namely the upper left corner of the unit square, and maximized the distance from a random predictor, namely the diagonal of the unit square).
A level of significance of .05 was considered statistically significant for all analyses if not otherwise mentioned. Two-sided tests were used in all analyses.
Results
Patients, treatment assignments, and transplantation outcomes
Clinical characteristics for the 71 patients included in the study are summarized in Table 1. Median age at transplantation was 40 years (range, 20-64 years). Forty-five patients (63%) received an allogeneic HSCT for acute leukemia or myelodysplastic syndrome, 10 (14%) for lymphoma, 12 (17%) for myeloma, 3 (4%) for myeloproliferative disease, and 1 (2%) for aplastic anemia. At the time of transplantation, 34 patients (48%) were in CR, 32 in partial remission or stable disease (45%), and 5 (7%) had a refractory or progressive disease. The choice of conditioning was dependent on the underlying disease, the recipient's age, and comorbidities.
Patients' characteristics at transplantation
| Baseline parameters . | All patients . |
|---|---|
| All patients | 71 (100) |
| Sex | |
| Male | 41 (57.8) |
| Female | 30 (43.2) |
| Median age of recipient, y (range) | 40.1 (20-64) |
| Median age of donor, y (range) | 39.8 (18.2-61.3) |
| Hematologic disease | |
| AML/ALL/MDS/MPD/AA | 25/11/9/3/1 |
| Lymphoma/myeloma | 10/12 |
| Disease status at transplantation | |
| CR | 34 (48) |
| PR or SD | 32 (45) |
| PD | 5 (7) |
| Conditioning regimen | |
| MAC | 25 (35) |
| RIC | 46 (65) |
| Donor | |
| Sibling | 43 (60) |
| Unrelated 10/10 | 21 (30) |
| Unrelated 9/10 | 7 (10) |
| Donor/recipient sex matching | |
| Female to male | 15 (21) |
| Other | 56 (79) |
| In vivo T depletion | |
| No | 39 (55) |
| Yes | 32 (45) |
| Risk of CMV | |
| Low | 20 (28.2) |
| Intermediate | 31 (43.6) |
| High | 20 (28.2) |
| HSC source | |
| BM | 21 (29.6) |
| PBSC | 50 (70.4) |
| Baseline parameters . | All patients . |
|---|---|
| All patients | 71 (100) |
| Sex | |
| Male | 41 (57.8) |
| Female | 30 (43.2) |
| Median age of recipient, y (range) | 40.1 (20-64) |
| Median age of donor, y (range) | 39.8 (18.2-61.3) |
| Hematologic disease | |
| AML/ALL/MDS/MPD/AA | 25/11/9/3/1 |
| Lymphoma/myeloma | 10/12 |
| Disease status at transplantation | |
| CR | 34 (48) |
| PR or SD | 32 (45) |
| PD | 5 (7) |
| Conditioning regimen | |
| MAC | 25 (35) |
| RIC | 46 (65) |
| Donor | |
| Sibling | 43 (60) |
| Unrelated 10/10 | 21 (30) |
| Unrelated 9/10 | 7 (10) |
| Donor/recipient sex matching | |
| Female to male | 15 (21) |
| Other | 56 (79) |
| In vivo T depletion | |
| No | 39 (55) |
| Yes | 32 (45) |
| Risk of CMV | |
| Low | 20 (28.2) |
| Intermediate | 31 (43.6) |
| High | 20 (28.2) |
| HSC source | |
| BM | 21 (29.6) |
| PBSC | 50 (70.4) |
Values are n (%) unless otherwise indicated. CMV reactivation risks were determined according to the donor (D) and recipient (R) serologies as follows: D−/R−, low risk; D+/R+ or D+/R−, intermediate risk; D−/R+, high risk.
AA indicates aplastic anemia; ALL, acute lymphoid leukemia; AML, acute myeloid leukemia, CR, complete remission; HSC, hematopoietic stem cell, MAC, myeloablative conditioning; MDS, myelodysplasic syndrome; MPD, myeloproliferative disease; PD, progressive disease; PR, partial remission; PBSC, peripheral blood stem cells; and RIC, reduced-intensity conditioning.
To summarize, patients in CR at transplantation who were younger than 50 years of age without comorbidities received a myeloablative conditioning regimen, either busulfan-cyclophosphamide (BU-CY) for acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) or 12 Gy total body irradiation/CY for acute lymphoid leukemia (25 patients). Refractory AML or MDS received a sequential regimen associating fludarabine (F)-cytarabine-idarubicine followed by BU-CY-ATG (8 patients). Patients older than 50 years of age or presenting with comorbidities received a reduced-intensity conditioning regimen, mostly F-BU-ATG for AML, MDS, or myeloproliferative syndromes; F-melphalan for myelomas; and rituximab-F-CY for lymphomas (38 patients). Altogether, 27 patients (38%) received ATG as part of the conditioning regimen. Forty-three patients (61%) received a graft from an HLA-identical family, and 28 (39%) from an unrelated donor, including 21 fully matched and 7 with 1 allelic class I mismatch. Hematopoietic stem cells were obtained from unmanipulated BM or peripheral blood in 30% and 70% of transplantations, respectively. The prevention of GVHD depended on the conditioning regimen and the level of matching between recipient and donor. Most patients received cyclosporine and methotrexate (66%) or mycophenolate mofetil (33%), with the adjunction of ATG in mismatched grafts when not included within the conditioning regimen (7%). The risk of CMV reactivation was low in 20 patients (28.2%), intermediate in 31 (43.6%), and high in 20 (28.2%).
At 3 years, OS was 66% (95% CI 52-77), and cumulative incidences of relapse and NRM were 18% (95% CI 11-36) and 25% (95% CI 17-43), respectively. The cumulative incidence of aGVHD was 58% (95% CI 47-70) at day 100, whereas the cumulative incidence of chronic GVHD was 41% (95% CI 36-66) at 3 years. Altogether, at 1 year, CMV reactivation had occurred in 45% (95% CI 35-48) of the patients.
Pretransplantation factors influencing iNKT cell reconstitution
Peripheral blood lymphocyte reconstitution was prospectively analyzed by flow cytometry on days 15, 30, 60, and 90 after allogeneic HSCT in the 71 patients. iNKT cells were specifically detected by the coexpression of CD3 and the PBS57-loaded CD1d-tetramer (Figure 1A). In most patients, iNKT cells became barely detectable after the conditioning (data not shown) and reappeared by day 15 after HSCT. Because iNKT cells represent a small proportion of T cells with potential immunomodulatory functions on conventional T cells, it is biologically more relevant to analyze the data in terms of ratio iNKT/T rather than absolute cell counts.
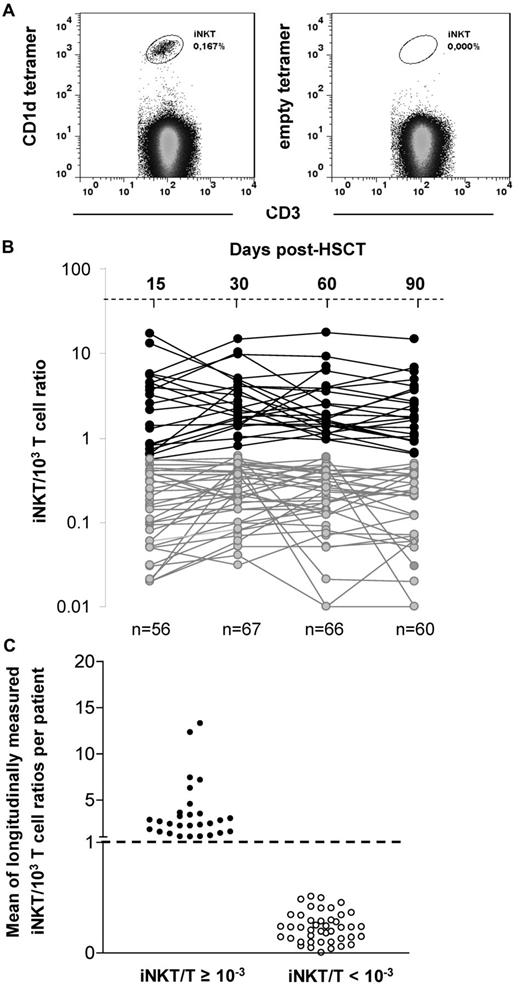
iNKT cell reconstitution after allogeneic transplantation. (A) iNKT cells are double stained by anti-CD3 and the PBS57-loaded CD1d-tetramer. Empty CD1d-tetramer was used as a negative control. (B) Evolution of the iNKT/T ratios between day 15 and 90 after HSCT (each line represents 1 patient) in the 71 analyzed patients (data were available for 56/71 patients on day 15, 67/71 on day 30, 66/71 on day 60, and 60/71 on day 90). Two patterns of reconstitution are observed: patients reaching a physiologic iNKT/T ratio ≥ 10−3 during the period of analysis and those with all iNKT/T ratios < 10−3. (C) Means of longitudinally measured iNKT/T ratios from days 15-90 of the 71 analyzed patients regarding whether they had reached the threshold of 10−3 during that period of time. Patients reaching the threshold at least once have also a mean of iNKT/T ratio > 10−3.
iNKT cell reconstitution after allogeneic transplantation. (A) iNKT cells are double stained by anti-CD3 and the PBS57-loaded CD1d-tetramer. Empty CD1d-tetramer was used as a negative control. (B) Evolution of the iNKT/T ratios between day 15 and 90 after HSCT (each line represents 1 patient) in the 71 analyzed patients (data were available for 56/71 patients on day 15, 67/71 on day 30, 66/71 on day 60, and 60/71 on day 90). Two patterns of reconstitution are observed: patients reaching a physiologic iNKT/T ratio ≥ 10−3 during the period of analysis and those with all iNKT/T ratios < 10−3. (C) Means of longitudinally measured iNKT/T ratios from days 15-90 of the 71 analyzed patients regarding whether they had reached the threshold of 10−3 during that period of time. Patients reaching the threshold at least once have also a mean of iNKT/T ratio > 10−3.
The usual iNKT/T cell ratio observed in healthy subjects varies from 0.1 × 10−3 to 10−2.35-37 The results of iNKT/T ratios measured on days 15 (range, days 13-20), 30 (range, days 25-35), 60 (range days, 55-65), and 90 (range, days 85-95) after HSCT in our patients are shown in Figure 1B. On the basis of the distribution of those ratios, we chose the threshold of 10−3 that appeared to discriminate 2 populations of patients: those reaching an iNKT/T ratio ≥ 10−3 between day 15 and day 90 after HSCT and those remaining at < 10−3 at all time points in the same period of time.
Among our 71 patients, 28 (39%) had at least 1 point above the ratio of 10−3 during the period of analysis, whereas 43 (61%) had all points below this threshold. Most patients reached the threshold of 10−3 between day 15 and 30, except for 1 patient, who reached the threshold by day 60. Among those, although all patients maintained their iNKT/T ratio ≥ 10−3 up to day 60, 6 had a decrease in their ratio on day 90. Nevertheless, the mean of longitudinally measured iNKT/T ratios was ≥ 10−3 in the patients with at least 1 point above the threshold of 10−3, whereas it was < 10−3 for all the others (Figure 1C). In absolute numbers, patients having an iNKT/T ratio ≥ 10−3 had also significantly greater peripheral counts of iNKT cells at each time point analyzed compared with those having an iNKT/T ratio < 10−3 at the same time points (Figure 2A). Thus, iNKT/T ratio and the threshold of 10−3 represent a reliable surrogate marker of global iNKT cell recovery in our series of allografted patients.
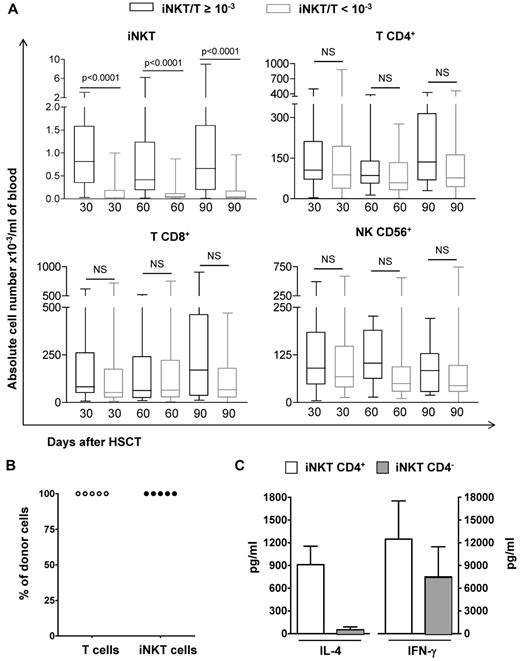
Absolute values T and NK cells according to the iNKT/T ratios, origin, and functionality of iNKT cells. (A) PBMCs from patients were harvested at 3 time points after HSCT and means (± SD) of absolute numbers of iNKT, T (CD4+ or CD8+), and NK (CD3−CD56+) cells are represented. The numbers of analyzed patients per time point were 25 on day 30, 23 on day 60, and 18 on day 90 for those with an iNKT/T ratio ≥ 10−3 and 42 on day 30, 43 on day 60, and 42 on day 90 for those with an iNKT/T ratio < 10−3. Although patients with an iNKT/T ratio ≥ 10−3 had also greater iNKT circulating cells in absolute numbers, they had similar T and NK reconstitution during the first 3 months after HSCT independent of their iNKT/T ratio (P < .0001, Mann-Whitney test). (B) CD4+ T or iNKT cells obtained after in vitro expansion were electronically sorted and genomic DNA extracted. In all patients analyzed, both CD4+ T and iNKT cells had a donor genotype. (C) Both CD4+ and CD4− iNKT cell subsets from patients independently of their iNKT/T ratios were expanded in culture before their electronic sorting. These cells were then stimulated and the levels of IL-4 and IFN-γ measured in the supernatants demonstrate their functionality. NS indicates nonsignificant.
Absolute values T and NK cells according to the iNKT/T ratios, origin, and functionality of iNKT cells. (A) PBMCs from patients were harvested at 3 time points after HSCT and means (± SD) of absolute numbers of iNKT, T (CD4+ or CD8+), and NK (CD3−CD56+) cells are represented. The numbers of analyzed patients per time point were 25 on day 30, 23 on day 60, and 18 on day 90 for those with an iNKT/T ratio ≥ 10−3 and 42 on day 30, 43 on day 60, and 42 on day 90 for those with an iNKT/T ratio < 10−3. Although patients with an iNKT/T ratio ≥ 10−3 had also greater iNKT circulating cells in absolute numbers, they had similar T and NK reconstitution during the first 3 months after HSCT independent of their iNKT/T ratio (P < .0001, Mann-Whitney test). (B) CD4+ T or iNKT cells obtained after in vitro expansion were electronically sorted and genomic DNA extracted. In all patients analyzed, both CD4+ T and iNKT cells had a donor genotype. (C) Both CD4+ and CD4− iNKT cell subsets from patients independently of their iNKT/T ratios were expanded in culture before their electronic sorting. These cells were then stimulated and the levels of IL-4 and IFN-γ measured in the supernatants demonstrate their functionality. NS indicates nonsignificant.
We first sought to analyze the association between pretransplantation baseline parameters with the posttransplantation iNKT cell recovery (assessed as the overall probability to have an iNKT/T ratio ≥ 10−3 on days 15, 30, 60, and/or 90) using GEE methodology (Table 2). In univariate analysis, recipient age younger than 45 years, age of the donor younger than 35 years, and the use of a family donor were associated with greater posttransplantation iNKT/T ratios. In multivariate analysis, an older recipient age was independently associated with a poor iNKT recovery (OR 0.21, 95% CI 0.07-0.59, P = .003), whereas a reduced-intensity conditioning regimen influenced favorably the posttransplantation iNKT reconstitution (OR 3.76, 95% CI 1.12-12.58, P = .031). An independent effect of the age of the donor was not observed.
Univariate and multivariate GEE regression analyses of iNKT/T ratios according to baseline parameters
| Baseline parameters . | Odds ratio (95% CI, modeling the probability that iNKT/T ratio ≥ 10−3 vs < 10−3) . | P . |
|---|---|---|
| Univariate analysis | ||
| Sex | ||
| Male | 1 | |
| Female | 0.52 (0.20-1.38) | .193 |
| Age of recipient, y | ||
| < 45 | 1* | |
| ≥ 45 | 0.30 (0.12-0.78)* | .013* |
| Age of donor, y† | ||
| < 35 | 1* | |
| ≥ 35 and < 45 | 0.20 (0.05-0.77)* | .020* |
| ≥ 45 | 0.43 (0.15-1.19) | .106 |
| Hematologic disease | ||
| AML/ALL/MDS/MPD/AA | 1 | |
| Lymphoma/myeloma | 0.59 (0.22-1.52) | .276 |
| Disease status at transplantation | ||
| CR | 1 | |
| PR or SD | 0.55 (0.21-1.44) | .227 |
| PD | 1.20 (0.19-7.39) | .841 |
| Conditioning regimen | ||
| MAC | 1 | |
| RIC | 2.39 (0.79-7.26) | .121 |
| Donor | ||
| Sibling | 1* | |
| Unrelated (10/10 or 9/10) | 0.29 (0.10-0.78)* | .015* |
| Donor/recipient sex matching | ||
| Female to male | 2.60 (0.90-7.51) | .075 |
| Other | 1 | |
| In vivo T depletion | ||
| No | 1 | |
| Yes | 1.46 (0.58-3.67) | .410 |
| CMV risk | ||
| Low | 1 | |
| Intermediate | 1.08 (0.36-3.22) | .880 |
| High | 1.22 (0.37-4.03) | .737 |
| Type of graft | ||
| BM | 1 | |
| PBMC | 3.48 (0.87-13.94) | .077 |
| Multivariate analysis | ||
| Age, y | ||
| < 45 | 1* | |
| ≥ 45 | 0.21 (0.07-0.59)* | .003* |
| Conditioning regimen | ||
| MAC | 1* | |
| RIC | 3.76 (1.12-12.58)* | .031* |
| Baseline parameters . | Odds ratio (95% CI, modeling the probability that iNKT/T ratio ≥ 10−3 vs < 10−3) . | P . |
|---|---|---|
| Univariate analysis | ||
| Sex | ||
| Male | 1 | |
| Female | 0.52 (0.20-1.38) | .193 |
| Age of recipient, y | ||
| < 45 | 1* | |
| ≥ 45 | 0.30 (0.12-0.78)* | .013* |
| Age of donor, y† | ||
| < 35 | 1* | |
| ≥ 35 and < 45 | 0.20 (0.05-0.77)* | .020* |
| ≥ 45 | 0.43 (0.15-1.19) | .106 |
| Hematologic disease | ||
| AML/ALL/MDS/MPD/AA | 1 | |
| Lymphoma/myeloma | 0.59 (0.22-1.52) | .276 |
| Disease status at transplantation | ||
| CR | 1 | |
| PR or SD | 0.55 (0.21-1.44) | .227 |
| PD | 1.20 (0.19-7.39) | .841 |
| Conditioning regimen | ||
| MAC | 1 | |
| RIC | 2.39 (0.79-7.26) | .121 |
| Donor | ||
| Sibling | 1* | |
| Unrelated (10/10 or 9/10) | 0.29 (0.10-0.78)* | .015* |
| Donor/recipient sex matching | ||
| Female to male | 2.60 (0.90-7.51) | .075 |
| Other | 1 | |
| In vivo T depletion | ||
| No | 1 | |
| Yes | 1.46 (0.58-3.67) | .410 |
| CMV risk | ||
| Low | 1 | |
| Intermediate | 1.08 (0.36-3.22) | .880 |
| High | 1.22 (0.37-4.03) | .737 |
| Type of graft | ||
| BM | 1 | |
| PBMC | 3.48 (0.87-13.94) | .077 |
| Multivariate analysis | ||
| Age, y | ||
| < 45 | 1* | |
| ≥ 45 | 0.21 (0.07-0.59)* | .003* |
| Conditioning regimen | ||
| MAC | 1* | |
| RIC | 3.76 (1.12-12.58)* | .031* |
AA indicates aplastic anemia; ALL, acute lymphoid leukemia; AML, acute myeloid leukemia, CI, confidence interval; CR, complete remission; GEE, generalized estimating equation; HSC, hematopoietic stem cell, invariant natural killer T/T cell; MAC, myeloablative conditioning; MDS, myelodysplasic syndrome; MPD, myeloproliferative disease; PD, progressive disease; PR, partial remission; PBSC, peripheral blood stem cells; and RIC, reduced-intensity conditioning.
Statistically significant.
Age of donor at time of transplantation was arbitrarily divided into tertiles.
T and NK immune reconstitution and iNKT cell functionality
We compared the reconstitution in absolute numbers of peripheral iNKT, CD4+ T, CD8+ T, and NK cells between patients reaching an iNKT/T ratio ≥ 10−3 and those who had not on days 30, 60, and 90 after HSCT (Figure 2A). At each time point, although patients with an iNKT/T ratio > 10−3 had also greater numbers of circulating iNKT cells, they had similar peripheral counts of T CD4+, T CD8+, and NK cells (Figure 2A). We did not detect any further correlation between iNKT/T ratios repeatedly measured and T CD4+, T CD8+, or CD56+ NK counts using GEE model (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Thus, iNKT recovery after allogeneic HSCT occurred independently of that of T and NK lymphocyte subsets. Chimerism analysis of ex vivo–expanded CD4+ iNKT cells could be performed in 5 patients from days 30 to 90 after transplantation. In all cases, iNKT cells detected after transplantation were derived from the donor (Figure 2B). In all analyzed cases, in vitro–expanded iNKT cells produced IL-4 and IFN-γ on stimulation, proving that they were functional (Figure 2C). Cytokine levels were similar to those previously reported for mature peripheral blood iNKT cells from healthy subjects,38 demonstrating that CD4+ iNKT cells were the major source of IL-4, in comparison with IFN-γ, which can be generated by both CD4+ and CD4− iNKT cell subsets (Figure 2C).
iNKT cell reconstitution and risks of GVHD and CMV reactivation
We then analyzed whether the iNKT/T ratios could be associated with the development of aGVHD. In univariate analysis, an iNKT/T ratio superior to 10−3 was significantly associated with the absence of aGVHD (hazard ratio [HR] 0.12, 95% CI 0.03-0.40, P < .001; Table 3). Apart from iNKT/T ratio, the use of an unrelated donor appeared as significantly associated with the development of grade 1-4 aGVHD in univariate analysis (HR 2.66, 95% CI 1.43-4.94, P = .001). In multivariate analysis, both iNKT/T ratio ≥ 10−3 and the use of a family donor remained independently associated with the absence of aGVHD (P = .001 and P = .006, respectively; Table 3).
Predictive factors of aGVHD (grade 1-4) development
| Baseline parameters . | Hazard ratio (95% CI; modeling the risk of aGVHD) . | P . |
|---|---|---|
| Univariate analysis | ||
| iNKT/T ratio | ||
| < 10−3 | 1* | |
| ≥ 10−3 | 0.12 (0.03-0.40)* | < .001* |
| Sex | ||
| Male | 1 | |
| Female | 1.13 (0.61-2.10) | .689 |
| Age of recipient, y | ||
| < 45 | 1 | |
| ≥ 45 | 1.63 (0.84-3.152) | .145 |
| Age of donor, y | ||
| < 35 | 1 | |
| ≥ 35 and < 45 | 1.81 (0.82-3.97) | .139 |
| ≥ 45 | 1.57 (0.74-3.32) | .235 |
| Disease status at transplantation | ||
| CR | 1 | |
| PR or SD | 1.59 (0.83-3.03) | .158 |
| PD | 1.92 (0.55-6.59) | .300 |
| Conditioning regimen | ||
| MAC | 1 | |
| RIC | 0.69 (0.36-1.28) | .250 |
| Donor | ||
| Sibling | 1* | |
| Unrelated (10/10 or 9/10) | 2.66 (1.43-4.94)* | .001* |
| Donor/recipient sex matching | ||
| Female to male | 0.79 (0.36-1.71) | .553 |
| Other | 1 | |
| In vivo T depletion | ||
| No | 1 | |
| Yes | 0.72 (0.38-1.35) | .307 |
| Risk of CMV | ||
| Low | 1 | |
| Intermediate | 1.00 (0.47-2.14) | .988 |
| High | 1.27 (0.57-2.84) | .554 |
| Type of graft | ||
| BM | 1 | |
| PBMC | 0.61 (0.32-1.18) | .147 |
| Multivariate analysis | ||
| iNKT/T ratio | ||
| < 10−3 | 1* | |
| ≥ 10−3 | 0.13 (0.03-0.44)* | .001* |
| Donor | ||
| Sibling | 1* | |
| Unrelated (10/10 or 9/10) | 2.36 (1.27-4.39)* | .006* |
| Baseline parameters . | Hazard ratio (95% CI; modeling the risk of aGVHD) . | P . |
|---|---|---|
| Univariate analysis | ||
| iNKT/T ratio | ||
| < 10−3 | 1* | |
| ≥ 10−3 | 0.12 (0.03-0.40)* | < .001* |
| Sex | ||
| Male | 1 | |
| Female | 1.13 (0.61-2.10) | .689 |
| Age of recipient, y | ||
| < 45 | 1 | |
| ≥ 45 | 1.63 (0.84-3.152) | .145 |
| Age of donor, y | ||
| < 35 | 1 | |
| ≥ 35 and < 45 | 1.81 (0.82-3.97) | .139 |
| ≥ 45 | 1.57 (0.74-3.32) | .235 |
| Disease status at transplantation | ||
| CR | 1 | |
| PR or SD | 1.59 (0.83-3.03) | .158 |
| PD | 1.92 (0.55-6.59) | .300 |
| Conditioning regimen | ||
| MAC | 1 | |
| RIC | 0.69 (0.36-1.28) | .250 |
| Donor | ||
| Sibling | 1* | |
| Unrelated (10/10 or 9/10) | 2.66 (1.43-4.94)* | .001* |
| Donor/recipient sex matching | ||
| Female to male | 0.79 (0.36-1.71) | .553 |
| Other | 1 | |
| In vivo T depletion | ||
| No | 1 | |
| Yes | 0.72 (0.38-1.35) | .307 |
| Risk of CMV | ||
| Low | 1 | |
| Intermediate | 1.00 (0.47-2.14) | .988 |
| High | 1.27 (0.57-2.84) | .554 |
| Type of graft | ||
| BM | 1 | |
| PBMC | 0.61 (0.32-1.18) | .147 |
| Multivariate analysis | ||
| iNKT/T ratio | ||
| < 10−3 | 1* | |
| ≥ 10−3 | 0.13 (0.03-0.44)* | .001* |
| Donor | ||
| Sibling | 1* | |
| Unrelated (10/10 or 9/10) | 2.36 (1.27-4.39)* | .006* |
Univariate and multivariate Cox proportional hazard ratio models considering iNKT/T ratio (repeatedly assessed on days 15, 30, 60, and 90 after HSCT) as a time-dependent variable.
CI indicates confidence interval; HSC, hematopoietic stem cell, MAC, myeloablative conditioning; PBSC, peripheral blood stem cells; and RIC, reduced-intensity conditioning.
Statistically significant.
Whereas no significant difference according to pretransplantation CMV risk category was observed, CMV reactivation occurred less frequently for patients reaching an iNKT/T ratio ≥ 10−3 (HR 0.35, 95% CI 0.14-0.86, P = .023). However, the development of aGVHD also was strongly associated with additional CMV reactivation (HR 2.48, 95% CI 1.21-5.09, P = .012), and the iNKT/T ratio could rather represent a surrogate marker for the risk of aGVHD and the subsequent risk of CMV reactivation.
The occurrence of chronic GVHD was not significantly affected by the iNKT cell reconstitution (cumulative incidence at 2 years of 36.3% in patients reaching at least once a iNKT/T ratio ≥ 10−3 vs 44.0% in those having all iNKT/T ratios < 10−3 until day 90, P = .53, Gray test).
iNKT reconstitution, relapse incidence, and final outcome
For survival analyses, 2 distinct groups of patients were considered: the “high iNKT/T” group (patients reaching at least once an iNKT/T ratio > 10−3) and the “low iNKT/T” group (patients never reaching such a ratio), as represented in Figure 1B. All analyses used a landmark approach with a landmark time set at 90 days. Median follow-up was 3.7 years. NRM was significantly lower in the high iNKT/T group compared with the low iNKT/T group (6.1% vs 36.7% at 2 years, respectively, P = .006; Figure 3A), whereas the incidence of relapse was similar between the 2 groups (13.9% vs 19.9% at 2 years, respectively, P = .426; Figure 3B). OS was significantly improved in the high iNKT/T group compared with the low iNKT/T group (90% vs 52% at 2 years, respectively, P = .002; Figure 3C). Analysis of death causes showed 2 deaths in the 28 patients in the high iNKT/T group (1 of infection and 1 of relapse), compared with 19 deaths within the 42 evaluable patients in the low iNKT/T group (14 of infection, 4 of relapse, and 1 of posttransplantation lymphoproliferative disease). Thus, poor iNKT cell reconstitution after allogeneic HSCT was associated with an increased mortality because of aGVHD and severe infections.
NRM, relapse incidence, and OS of patients according to their iNKT reconstitution in landmark analyses at day 90. (A) Cumulative incidence of NRM was significantly increased in patients with iNKT/T ratios < 10−3 (low iNKT/T group; P = .009, Gray test). (B) The cumulative incidence of relapse was similar between patients whether or not they reached iNKT/T ratios ≥ 10−3 (high vs low iNKT/T group; P = .770, Gray test). (C) OS was significantly prolonged for patients reaching iNKT/T ratio ≥ 10−3 (high iNKT/T group; P = .002, logrank test). NS indicates nonsignificant. Only 1 patient died before day 90 and was therefore not included in the landmark analysis.
NRM, relapse incidence, and OS of patients according to their iNKT reconstitution in landmark analyses at day 90. (A) Cumulative incidence of NRM was significantly increased in patients with iNKT/T ratios < 10−3 (low iNKT/T group; P = .009, Gray test). (B) The cumulative incidence of relapse was similar between patients whether or not they reached iNKT/T ratios ≥ 10−3 (high vs low iNKT/T group; P = .770, Gray test). (C) OS was significantly prolonged for patients reaching iNKT/T ratio ≥ 10−3 (high iNKT/T group; P = .002, logrank test). NS indicates nonsignificant. Only 1 patient died before day 90 and was therefore not included in the landmark analysis.
In univariate analysis, factors associated with an improved OS were iNKT/T ratio ≥ 10−3, recipient age younger than 45 years, the use of a family donor, and the absence of T depletion (Table 4). In multivariate analysis, only the iNKT/T reconstitution and the use of a family donor correlated with an improved outcome (HR 0.19, 95% CI 0.04-0.83, P = .028 for iNKT/T ratio ≥ 10−3 vs < 10−3; Table 4).
Univariate and multivariate OS analyses of iNKT/T ratio according to baseline parameters
| Baseline parameters . | Univariate analysis* . | Multivariate analysis* . | ||
|---|---|---|---|---|
| 2-y OS, % (95% CI) . | P† . | HR (95% CI) . | P‡ . | |
| iNKT/T ratio* | ||||
| < 10−3 | 52 (35-69)§ | .002§ | 1§ | .028§ |
| ≥ 10−3 | 90 (76-100)§ | 0.19 (0.04-0.83)§ | ||
| Sex | ||||
| Male | 66 (48-83) | .546 | ||
| Female | 66 (48-84) | |||
| Age of recipient, y | ||||
| < 45 | 81 (65-98)§ | .037§ | ||
| ≥ 45 | 56 (39-74)§ | |||
| Age of donor, y | ||||
| < 35 | 72 (48-86) | .475 | ||
| ≥ 35 and < 45 | 63 (34-82) | |||
| ≥ 45 | 64 (38-81) | |||
| Hematologic disease | .573 | |||
| ALL/AML/AA/MPD | 66 (51-82) | |||
| Lymphoma/myeloma | 70 (50-90) | |||
| Disease status at transplantation | ||||
| CR | 68 (49-86) | .173 | ||
| PR or SD | 66 (48-84) | |||
| PD | 53 (4-100) | |||
| Conditioning regimen | ||||
| MAC | 72 (53-91) | .528 | ||
| RIC | 62 (46-79) | |||
| Donor | ||||
| Sibling | 80 (67-93)§ | .002§ | 1§ | .031§ |
| Unrelated (10/10 or 9/10) | 43 (21-65)§ | 2.78 (1.09-7.10)§ | ||
| Donor | ||||
| Female to male | 60 (32-88) | .495 | ||
| Other | 68 (54-82) | |||
| In vivo T depletion | ||||
| No | 78 (63-92)§ | .033§ | ||
| Yes | 51 (31-72)§ | |||
| Risk of CMV | ||||
| Low | 79 (57-100) | .128 | ||
| Intermediate | 71 (54-88) | |||
| High | 40 (12-69) | |||
| Type of graft | ||||
| BM | 59 (36-83) | .331 | ||
| PBSC | 69 (54-84) | |||
| Baseline parameters . | Univariate analysis* . | Multivariate analysis* . | ||
|---|---|---|---|---|
| 2-y OS, % (95% CI) . | P† . | HR (95% CI) . | P‡ . | |
| iNKT/T ratio* | ||||
| < 10−3 | 52 (35-69)§ | .002§ | 1§ | .028§ |
| ≥ 10−3 | 90 (76-100)§ | 0.19 (0.04-0.83)§ | ||
| Sex | ||||
| Male | 66 (48-83) | .546 | ||
| Female | 66 (48-84) | |||
| Age of recipient, y | ||||
| < 45 | 81 (65-98)§ | .037§ | ||
| ≥ 45 | 56 (39-74)§ | |||
| Age of donor, y | ||||
| < 35 | 72 (48-86) | .475 | ||
| ≥ 35 and < 45 | 63 (34-82) | |||
| ≥ 45 | 64 (38-81) | |||
| Hematologic disease | .573 | |||
| ALL/AML/AA/MPD | 66 (51-82) | |||
| Lymphoma/myeloma | 70 (50-90) | |||
| Disease status at transplantation | ||||
| CR | 68 (49-86) | .173 | ||
| PR or SD | 66 (48-84) | |||
| PD | 53 (4-100) | |||
| Conditioning regimen | ||||
| MAC | 72 (53-91) | .528 | ||
| RIC | 62 (46-79) | |||
| Donor | ||||
| Sibling | 80 (67-93)§ | .002§ | 1§ | .031§ |
| Unrelated (10/10 or 9/10) | 43 (21-65)§ | 2.78 (1.09-7.10)§ | ||
| Donor | ||||
| Female to male | 60 (32-88) | .495 | ||
| Other | 68 (54-82) | |||
| In vivo T depletion | ||||
| No | 78 (63-92)§ | .033§ | ||
| Yes | 51 (31-72)§ | |||
| Risk of CMV | ||||
| Low | 79 (57-100) | .128 | ||
| Intermediate | 71 (54-88) | |||
| High | 40 (12-69) | |||
| Type of graft | ||||
| BM | 59 (36-83) | .331 | ||
| PBSC | 69 (54-84) | |||
AA indicates aplastic anemia; ALL, acute lymphoid leukemia; AML, acute myeloid leukemia, CI, confidence interval; CR, complete remission; HR, hazard ratio; HSC, hematopoietic stem cell; iNKT/T, invariant natural killer T/T cell; MAC, myeloablative conditioning; MDS, myelodysplasic syndrome; MPD, myeloproliferative disease; OS, overall survival; PD, progressive disease; PR, partial remission; PBSC, peripheral blood stem cells; and RIC, reduced-intensity conditioning.
Univariate and multivariate landmark analyses were conducted with a landmark time set up at day 90 when NKT reconstitution was fully determined.
Using the logrank test.
Using theCox proportional hazard ratio model. Only 1 patient died before day 90 and was therefore not included in the landmark analysis.
Statistically significant.
Predictive value of day 15 iNKT/T ratio on the occurrence of aGVHD
To define a useful tool for clinical practice, we sought to determine whether a high iNKT/T ratio early after transplantation could predict the absence of aGVHD. Indeed, the median time when patients reached an iNKT/T ratio > 10−3 was 24 days, whereas aGVHD occurred at median time on day 20 (range,12-80) post-HSCT in our series of patients.
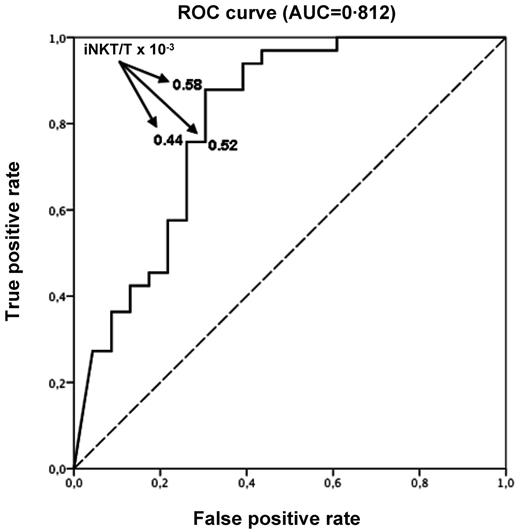
Fifty-six patients of 71 had an available iNKT/T ratio on day 15 after HSCT. Missing data were mainly the result of low numbers of T cells impairing the measurement of a reliable iNKT/T ratio at that early time point. Using ROC curve, we found that day 15 iNKT/T ratio could efficiently discriminate the risk of aGVHD with an AUC of 0.812 (95% CI 0.536-1.000; Figure 4). A cut-off iNKT/T ratio of 0.58 × 10−3, providing the best balance of sensitivity and specificity, yield an 87.9% true-positive rate and a 30.5% false-positive rate. Using a logistic regression model, we found that day 15 iNKT/T ratio > 0.58 × 10−3 was therefore associated with a 94% reduced chance of developing aGVHD (OR 0.06, 95% CI 0.01-0.23). Using this cut-off point, we found that only 4 of 20 patients (20.0%) with an early iNKT recovery experienced aGVHD, whereas 29 of 36 (80.5%) did so if the iNKT/T ratio was < 0.58 × 10−3.
ROC curve with day 15 iNKT/T ratio. Day 15 iNKT/T ratio can discriminate the risk of aGVHD (AUC = 0.812). An AUC of 1.0 indicates perfect discrimination, whereas an area of 0.5 indicates that the test discriminates no better than chance. An iNKT/T cut-off ratio equal to 0.58 × 10−3 at day 15 yields the best balance between specificity and sensitivity (87.9% true-positive rate and 30.5% false-positive rate).
ROC curve with day 15 iNKT/T ratio. Day 15 iNKT/T ratio can discriminate the risk of aGVHD (AUC = 0.812). An AUC of 1.0 indicates perfect discrimination, whereas an area of 0.5 indicates that the test discriminates no better than chance. An iNKT/T cut-off ratio equal to 0.58 × 10−3 at day 15 yields the best balance between specificity and sensitivity (87.9% true-positive rate and 30.5% false-positive rate).
Discussion
We report the first evidence for a relationship between iNKT cell reconstitution and the clinical evolution of allogeneic HSCT in adult patients. Our results show that a fast and enhanced replenishment of peripheral donor–derived iNKT cells (analyzed in terms of iNKT/T ratios and in absolute values) provides a new posttransplantion predictive marker of reduced risk of aGVHD associated with a preserved GVL effect. Most importantly, iNKT reconstitution appeared as an independent predictive factor of OS, as well as the use of a family donor.
Some arguments support a causative effect of iNKT cells on the absence of aGVHD rather than a consequence. First, iNKT cell ratios and counts increased shortly after transplantation, before and independently of conventional T- and NK-cell reconstitution. Second, a high iNKT/T ratio, as earlier as on day 15 after transplantation, can predict the absence of aGVHD occurring, on average, 2 weeks later.
The reasons for a better iNKT cell reconstitution in some allografted patients remain to be determined. Two factors independently influenced this reconstitution in our series of patients: younger recipient's age and the use of a reduced-intensity conditioning. It is known that iNKT cell counts decline over age.39 Although patients reaching greater iNKT/T ratios were younger, their pretransplantation levels of peripheral iNKT cells were not significantly greater (data not shown), which might be because of an altered iNKT cell pool after treatment of a hematologic condition. It is therefore difficult to assess the role of recipient microenvironment in the expansion of iNKT cells after transplantation. In addition, it is possible that high doses of chemotherapy used in myeloablative conditionings have greater toxicity on the microenvironment required for the survival and expansion of iNKT lymphocytes.
Although we did not observe a correlation between donor age and iNKT cell reconstitution in multivariate analysis, another possible explanation is that this reconstitution is dependent on the donor graft content. We did not find any significant difference in terms of CD34+ and total T-cell graft doses between the patients reaching or not reaching greater ratios of iNKT/T cells (data not shown). No other cell type was analyzed in the graft; thus, we could not rely the number of iNKT cell transferred and their potential of reconstitution in the host. However, favoring this hypothesis, another group recently has shown that peripheral blood stem cell grafts containing greater CD4− iNKT cell doses were associated with a reduced risk of aGVHD.40 It is noteworthy that these authors did not observe any correlation between donor age and neither the graft content in total or subtypes of iNKT cells nor the occurrence of aGVHD after HSCT.40 Their results are therefore in line with the absence of independent effect of donor age on the occurrence of GVHD in our study.
There is increasing evidence for a role of dendritic cells (DCs) in the regulation of the GVH and GVL effects after allogeneic HSCT.41 Because iNKT cell survival and expansion require the presence of antigen-presenting cells such as DCs, their reconstitution in the host may depend on host or donor DC number and functionality as well. Thus, further explorations of graft content in DC, T-, and iNKT cell subtypes are therefore warranted to provide some clues of the complexity of this cell network for the posttransplantion immune reconstitution and regulation of GVHD.
Our results are in agreement with a previous report in which the authors suggested a beneficial effect of iNKT cells in reducing the risk of aGVHD after allogeneic HSCT conditioned with total lymphoid irradiation (TLI).42-44 In this study, the authors implicated the role of recipient-derived iNKT cells on the basis of the mechanistic results obtained in their corresponding mouse model.29 In our patients, however, chimerism analyses revealed that iNKT cells emerging after allogeneic HSCT were of donor origin. Donor iNKT cells also have been implicated in the results reported by Chaidos et al.40 The conditioning used in these studies is a plausible reason for this discrepancy because recipient iNKT cells are radioresistant and might better survive among T cells in patients conditioned with TLI. This phenomenon is unlikely to occur in conventional conditioning, as used by us and Chaidos et al,40 which uniformly depletes all T cells, at least in the blood, as observed in our series of patients on day 0 after transplantation (data not shown).
In the context of pediatric haploidentical T cell–depleted allogeneic HSCTs, another group has recently reported greater iNKT/T cell ratios by day 100 after transplantation in the nonrelapsing patients compared with those who relapsed within the first 18 months after transplantation.45 Our study, even if not statistically significant, supports this observation because patients with an improved iNKT/T ratio have a trend to a lower risk of relapse (13.9% in the high iNKT/T group vs 19.9% in the low iNKT/T group).
The mechanisms of action of iNKT cells in regulating the GVH/GVL balance in humans remained to be determined. In the mouse model conditioned by TLI, host iNKT cells inhibit GVHD via their production of IL-4, driving T cells toward a Th2 response and the expansion of Tregs.46 In human patients, increased Th1 cytokine levels are associated with the development of aGVHD,47,48 whereas IL-10 polymorphism might protect against GVHD.49 However, whether a Th2 cytokine pattern can be associated with an improved OS and the potential role of iNKT cells in such immunologic environment remain to be demonstrated in human allogeneic HSCT. In our series of patients, iNKT cells arising after HSCT are fully mature in terms of CD161 expression (data not shown) and of in vitro production of IFN-γ and IL-4. Consequently, these cells are likely to act as immunomodulatory cells.
Similar to previous reports,7,8 we have observed decreased peripheral blood Treg counts in patients developing aGVHD (M.-T.R., M.B., O.H., unpublished data, April 2012). However, in contrast to iNKT cells, reduced Treg frequency does not predict the occurrence of aGVHD but likely represents an immunologic consequence of its development. In experimental models, iNKT cells were shown to enhance the expansion of Tregs through IL-2 production.50 We cannot exclude a possible cross-talk between iNKT cells and Tregs in the protection against human aGVHD, but further studies are necessary to solve this point.
In our group of patients, iNKT reconstitution was not correlated with the development of chronic GVHD, emphasizing different mechanisms of acute versus chronic GVHD. This might, at least in part, explain the preservation of the GVL effect.
In conclusion, our findings suggest for the first time a positive correlation between iNKT cell reconstitution, reduced risk of GVHD, preserved GVL effect and improved posttransplantation OS of adult patients undergoing allogeneic HSCT. Although our data are determined on the basis of a relatively small cohort and that further analyses are required on a larger and more homogeneous group of patients to define the best early iNKT/T ratio and time point discriminating the posttransplantation evolution, our results provide the proof of concept that iNKT cells represent an important regulatory T-cell subset implicated in the immunology of allogeneic HSCT. Early iNKT/T-cell ratio could be in the future used as a new parameter to adapt the therapeutic interventions on GVHD, and the manipulation of donor iNKT cells might represent an attractive strategy to improve the results of allogeneic HSCT.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Elke Schneider for critical review of the manuscript and J. Mégret and C. Garcia for cell sorting. They thank the National Institutes of Health Tetramer Core Facility for providing CD1d tetramer reagents, the European Erasmus Program for training master students, the clinical investigation center (CIC) of Necker Hospital for their implication in clinical studies, the “Ligue Nationale contre le Cancer,” the APHP, the “Cancéropole d'Ile de France,” and the “Institut National du Cancer” for supportive grants.
Authorship
Contribution: M.-T.R., E.B., O.H., and M.L.-d.-M. designed the study; M.-T.R., L.-M.T., M.B., P.M., and M.L.-d.-M. collected the data; M.-T.R., T.C., F.S., A.M., D.S., A.B., B.V., and O.H. cared for the patients; S.C.-Z., M.C.-C., and M.L.-d.-M. provided reagents; M.-T.R., E.B., M.B., P.M., O.H., and M.L.-d.-M. analyzed the data; and M.-T.R., E.B., T.C., M.D., O.H., and M.L.d.-M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Marie-Thérèse Rubio, Hematology Department, Saint Antoine Hospital, 184, rue du Faubourg Saint Antoine, 75012 Paris, France; e-mail: mt_rubio@hotmail.com; Pr Olivier Hermine, Hematology Department, Necker Hospital, 149-161 rue de Sèvres, 75743 Paris Cedex 15, France; e-mail: ohermine@gmail.com; or Dr Maria Leite-de-Moraes, Unité Mixte de Recherche 8147, Centre National de la Recherche Scientifique, Faculté de Médecine René Descartes, Paris V, Hôpital Necker, 161 rue de Sèvres, 75015, Paris, France; e-mail: maria.leite-de-moraes@parisdescartes.fr.
References
Author notes
L.M.-T., E.B., O.H., and M.L.-d.-M. contributed equally to this work.