Abstract
For a long time, blood coagulation and innate immunity have been viewed as interrelated responses. Recently, the presence of leukocytes at the sites of vessel injury has been described. Here we analyzed interaction of neutrophils, monocytes, and platelets in thrombus formation after a laser-induced injury in vivo. Neutrophils immediately adhered to injured vessels, preceding platelets, by binding to the activated endothelium via leukocyte function antigen-1–ICAM-1 interactions. Monocytes rolled on a thrombus 3 to 5 minutes postinjury. The kinetics of thrombus formation and fibrin generation were drastically reduced in low tissue factor (TF) mice whereas the absence of factor XII had no effect. In vitro, TF was detected in neutrophils. In vivo, the inhibition of neutrophil binding to the vessel wall reduced the presence of TF and diminished the generation of fibrin and platelet accumulation. Injection of wild-type neutrophils into low TF mice partially restored the activation of the blood coagulation cascade and accumulation of platelets. Our results show that the interaction of neutrophils with endothelial cells is a critical step preceding platelet accumulation for initiating arterial thrombosis in injured vessels. Targeting neutrophils interacting with endothelial cells may constitute an efficient strategy to reduce thrombosis.
Introduction
After vessel injury, a rapid accumulation of platelets and activation of the blood coagulation cascade are critical steps to prevent blood loss. In addition, blood polymorphonuclear neutrophils (PMNs) play a major role in limiting intrusion of microorganisms into the bloodstream. For a long time, different observations have linked blood coagulation and innate immunity. In 1882, Bizzozero first observed that the formation of a thrombus involved both platelets and leukocytes.1 More recently, the presence of leukocytes accumulating at the sites of vessel injury has been described.2,3 Clinical studies have shown that traditional vascular risk factors are associated with leukocyte activation and may predispose to thrombogenesis.4,5 PMNs may contribute to fibrin generation directly by exposing active tissue factor (TF)6 and/or indirectly by inactivating the major TF inhibitor, TF pathway inhibitor (TFPI).2 PMNs may also locally assemble proteins of the factor XII–driven contact pathway.7 Recent advances in digital microscopy make it possible to observe, in real time, the formation of a thrombus after a vessel injury in living mice.8 In this model of laser-induced injury, the subendothelial matrix is not exposed to the blood circulation.9 Thrombin is locally generated, and platelet-rich thrombi are formed after activation of the endothelial cells.10 von Brühl et al have recently showed the importance of monocyte, PMN, and platelet cross-talk in a mouse model of deep vein thrombosis (DVT).3 In this model, neutrophils are indispensable for propagation of the clotting cascade by binding factor XII (FXII) and by supporting its activation through the release of neutrophil extracellular traps (NETs).11 After exposure of the subendothelial matrix, neutrophils may, by interacting with platelets, inactivate TFPI thus leading to the activation of the TF-dependent coagulation cascade.2 Vascular smooth muscle–derived TF is also critical for arterial thrombosis in mice.12 After a laser-induced injury, a previous study suggested that monocyte-derived microparticles contribute to thrombus formation in arteries by delivering TF to the site of injury and interacting with accumulating platelets.13,14 However, the relative contribution of neutrophils and monocytes in microvascular thrombosis remains unclear. Using the laser-injury system with a high-definition, high-speed camera, we analyzed interaction of neutrophils, monocytes, and platelets in thrombus formation in the microvasculature of mice. Our results demonstrate that neutrophils, by binding to the vessel wall, are the main source of blood-borne TF in the early phase of thrombus formation.
Methods
Mice
Wild-type C57BL/6J mice were from Janvier Elevage. CX3CR1GFP C57BL/6J mice were obtained from The Jackson Laboratory. FXII−/− mice and low TF mice have been previously described.15-17 All animal care and experimental procedures were performed as recommended by the European Community guidelines and approved by the French Ministry of Agriculture (agreement no. 13.382; L.P.-D.).
Antibodies and reagents
The rat anti–mouse CD41 antibody (clone MWReg30; 0.2 μg/g mouse weight) and R300 antibody used to deplete circulating platelets18 were obtained from Emfret. Alexa Fluor 488 rat anti–mouse, lysosomal-associated membrane protein 1 (LAMP-1; CD107a) antibody (clone 1D4B; 0.25 μg/g mouse weight) and Alexa Fluor 647 anti–mouse CD54 (ICAM-1) antibody (clone YN1/1.7.4; 0.5 μg/g mouse weight) were from Biolegend (Ozyme Biolegend). PE rat anti–mouse Ly-6G antibody (clone 1A8; 0.1 μg/g mouse weight), purified rat anti–mouse Ly-6G and Ly-6C antibody (clone RB6-8C5), Alexa Fluor 647 rat anti–mouse CD11b (Mac-1) antibody (clone M1/70; 1 μg/g mouse weight), blocking rat monoclonal anti–mouse P-selectin antibody (clone RB40.34), purified hamster anti–mouse ICAM-1 antibody (clone 3E2), rat anti–mouse CD11a (leukocyte function antigen-1 [LFA-1]) antibody (clone M17/4), allophycocyanin rat anti–mouse CD45 antibody (clone 30-F11), rat anti–mouse TER-119 antibody (clone TER-119; 0.59 μg/g mouse weight), and rat IgG2a isotype control were from BD Biosciences. Blocking anti–mouse ICAM-1 and LFA-1 antibodies were, respectively, used at 4 μg/g and 2 μg/g of mouse weight. Isolectin B4 used to label the endothelial wall,19 DIO [iodide of (dodecyl-4 aminostyrile)-4 N-methylpyridium] was obtained from Life Technologies. The mouse mAb directed against human, rat, and mouse TF (ab104513) was from Abcam. 1H1 antibody directed against mouse TF was obtained from Dr Daniel Kirchhofer (University of North Carolina, Chapel Hill, NC).20 A mouse anti–human fibrin II γ-chain antibody (clone NYBT2G1) was from Accurate Chemical.
Fab fragments from the anti-CD41 antibody were generated using the Immuno-Pure Fab Preparation Kit (Pierce Thermofisher). Fab fragments, antibodies, and control isotypes were conjugated to dye light Fluor 488 or 647 according to the manufacturer's instruction (Pierce Thermofisher). Prostaglandin I2 was obtained from Merck. Apyrase grade VI, saponin, and BSA were purchased from Sigma-Aldrich. Corn trypsin inhibitor was obtained from Cryopep.
Flow cytometry
Blood was collected from mice in a citrate solution (ACD: 85mM trisodium citrate, 67mM citric acid, 111.5mM glucose, pH 4.5) in the presence of 0.5mM prostacyclin and 0.02 U/mL apyrase as previously described.21 Analyses of blood and platelet-rich plasma samples were performed on a flow cytometer (Gallios; Beckman Coulter) using appropriate antibodies to label white blood cells.22
Isolation of leukocytes
Leukocytes were extracted from spleen of wild-type mice as previously described by StemCell Technologies. Isolated leukocytes were counted in a Malassez cell and labeled with DIO according to the manufacturer's instruction (Vybrant Kit; Life Technologies). Purified labeled cells were resuspended in saline buffer at a concentration of 1 × 109 cells/mL and were infused in the blood of a recipient mouse at a concentration of 2 × 106 cells/mouse.
Isolation of neutrophils
Neutrophils from bone marrow or peripheral blood were isolated as previously described23 using GR-1–coupled magnetic beads (anti–Ly-6G Microbead kit; Milteny Biotec).
Depletion of neutrophils
Depletion of neutrophils from wild-type mice was performed by intravenous injection of 5 μg/g mouse of rat anti–Ly-6G/C (RB6-8C5; eBioscience) as previously described.24 Control mice were injected with PBS. Neutropenia was evaluated 24 hours after injection by flow cytometry using a PE-conjugated anti–mouse neutrophil mAb.
Fluorescence microscopy
Purified neutrophils were washed with PBS and fixed for 30 minutes at 4°C in PBS containing 2% paraformaldehyde. Once fixed, cell suspensions were cytocentrifugated for 10 minutes at 125g in a shandom cytospin 4 (Thermo Fisher Scientific). Cells were permeabilized using PBS containing 0.3% saponin for 20 minutes. Then, cells were blocked for 20 minutes at 4°C with BSA 1%, saponin 0.3% in PBS buffer. Cells were incubated at 4°C for 2 hours with the appropriate dilution of antibodies and 0.3% saponin, followed by incubation with an Alexa 488–conjugated secondary antibody. Between each step, cells were rinsed with PBS containing 0.3% saponin and were observed on a microscope (Nikon Eclipse TE2000-U).
Western blotting
Western blotting was performed after SDS polyacrylamide (11%) gel electrophoresis. Proteins were transferred onto a nitrocellulose membrane using an iBlot system (Life Technologies) according to the manufacturer's instruction. Transfer was verified by staining membranes with Ponceau red. Detection of the labeled proteins was performed by chemiluminescence using ECL from GE Healthcare.
Intravital microscopy
Intravital videomicroscopy of the cremaster muscle microcirculation was performed as previously described8 using an Intelligent Imaging Innovations system. Briefly, mice were preanesthetized with intraperitoneal ketamine (125 mg/kg; Panpharma), xylazine (12.5 mg/kg; Bayer), and atropine (0.25 mg/kg; Lavoisier). A tracheal tube was inserted and the mouse maintained at 37°C on a thermo-controlled rodent blanket. To maintain anesthesia, pentobarbital (CEVA) was administered through a cannula placed in the jugular vein. After the scrotum was incised, the testicle and surrounding cremaster muscle were exteriorized onto an intravital microscopy tray. The cremaster preparation was superfused with a thermo-controlled (37°C) bicarbonate-buffered saline throughout the experiment. Microvessel data were obtained using an Olympus AX microscope with a 60× or 100× 0.9 NA water-immersion objective. The fluorescence microscopy system has previously been described.25 Digital images were captured with a Cooke Sensicam CCD camera in 640 × 480–pixel format or with a CoolSnapEZ HD camera in 1392 × 1040–pixel format.
Laser-induced injury
Antibodies or exogenously labeled mouse leukocytes were infused through the jugular vein into the circulation of an anesthetized mouse. Vessel wall injury was induced with a nitrogen dye laser (Micropoint; Photonics Instruments) focused through the microscope objective, parafocal with the focal plane, and aimed at the vessel wall.9 Typically, 1 or 2 pulses were required to induce vessel wall injury. For all the experiments performed involving injection through the jugular vein of antibodies or cells, 3 to 5 mice were studied for each condition and a minimum of 10 thrombi induced per mouse. In all experiments, new thrombi were formed upstream of earlier thrombi to avoid any contribution from thrombi generated earlier. There were no characteristic trends in thrombus size or thrombus composition in sequential thrombi generated in a single mouse during an experiment. Image analysis was performed using Slidebook (Intelligent Imaging Innovations). Fluorescence data were captured digitally at up to 50 frames per second and analyzed as previously described to determine the median of fluorescent intensity signal overtime.8
Statistics
Significance was determined by the Wilcoxon rank-sum test for the in vivo experiments.8 Differences were considered significant at P < .05.
Results
Leukocytes accumulate at the site of arterial injury independent of platelets
In the second after vessel injury, cells of 10 to 20 μm in diameter were seen to accumulate at the injury site. These cells were also detectable 3 to 5 seconds after the injury when platelets started to accumulate, as well as 80 to 120 seconds postinjury when the thrombus was at its maximal size and 3 minutes later when it was stabilized (Figure 1A). Fluorescently labeled leukocytes infused into mice were detected at the site of injury with similar kinetics (Figure 1B). In addition, a fluorescent signal was observed in the second after the injury when a Mac-1 (macrophage 1 antigen, CD11b) but not an irrelevant antibody (Figure 1C) was used. In addition, when the red blood cell–specific (RBC) TER-119 antibody was infused, RBCs were detected in the circulating blood but not at the site of thrombus (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The kinetics of Mac-1–positive cells indicates that leukocytes rapidly accumulate at the site of laser-induced injury in vivo (supplemental Figure 1B). After the depletion of the circulating platelets, no signal corresponding to platelet accumulation was detected at the site of injury. However, the kinetics of leukocyte accumulation were identical in the presence or the absence of platelets (Figure 1D). We concluded that leukocytes accumulated before platelets at the site of laser-induced injury by interacting with activated endothelial cells.
Real-time imaging of leukocyte accumulation at the site of injury in wild-type mice. (A) Representative images of cells (white arrows) accumulating at the site of injury (n = 32; 3 mice). (B) Exogenously-labeled leukocytes (blue) were detected at the site of injury (top panel). (C) Accumulation of Mac-1 (blue), but not the irrelevant antibody, was detected at the site of thrombus formation (white arrows; middle panel). Images are representative of 32 thrombi performed in 3 mice for each condition. (D) Detection of leukocytes before (top panel) or after (bottom panel) depletion of platelets. The graphs depict the median of the maximal integrated fluorescence intensity of CD41 (left panel) and Mac-1 (right panel) corresponding to platelets and leukocytes accumulation, respectively (31 thrombi; 3 mice); *P < .05.
Real-time imaging of leukocyte accumulation at the site of injury in wild-type mice. (A) Representative images of cells (white arrows) accumulating at the site of injury (n = 32; 3 mice). (B) Exogenously-labeled leukocytes (blue) were detected at the site of injury (top panel). (C) Accumulation of Mac-1 (blue), but not the irrelevant antibody, was detected at the site of thrombus formation (white arrows; middle panel). Images are representative of 32 thrombi performed in 3 mice for each condition. (D) Detection of leukocytes before (top panel) or after (bottom panel) depletion of platelets. The graphs depict the median of the maximal integrated fluorescence intensity of CD41 (left panel) and Mac-1 (right panel) corresponding to platelets and leukocytes accumulation, respectively (31 thrombi; 3 mice); *P < .05.
LFA-1–expressing leukocytes interact with ICAM-1 expressed on injured vascular wall
Recently, Atkinson et al determined that endothelial cells were activated after the use of a dye laser.26 We confirmed these results and found that both LAMP-1 (CD107a) and ICAM-1 (CD54) were detected on endothelial cells immediately after the injury (supplemental Figure 1C). This laser-induced activation of the endothelium was a prerequisite for the interaction of leukocytes because the fluorescently labeled circulating leukocytes did not interact with resting endothelium in vivo. Blocking ICAM-1 abolished the accumulation of leukocytes at the site of laser-induced injury (Figure 2A). Similar results were observed after blocking the ICAM-1 ligand, LFA-1 (CD11a), expressed by leukocytes (Figure 2B).
Leukocyte accumulation is dependent onLFA-1/ ICAM-1 interactions. Leukocyte (blue) accumulation at site of injury (white arrow) in absence (top panel) or presence (bottom panel) of (B) ICAM-1 or (C) LFA-1 blocking antibody. Graph represents medians of Mac-1–integrated fluorescence intensity before (upper curve, 33 thrombi; 3 mice) and after (lower curve, 31 thrombi; 3 mice) infusion of (B) ICAM-1 or (C) LFA-1–blocking antibody.
Leukocyte accumulation is dependent onLFA-1/ ICAM-1 interactions. Leukocyte (blue) accumulation at site of injury (white arrow) in absence (top panel) or presence (bottom panel) of (B) ICAM-1 or (C) LFA-1 blocking antibody. Graph represents medians of Mac-1–integrated fluorescence intensity before (upper curve, 33 thrombi; 3 mice) and after (lower curve, 31 thrombi; 3 mice) infusion of (B) ICAM-1 or (C) LFA-1–blocking antibody.
Neutrophils but not monocytes accumulate at the site of injury
To determine whether monocytes were rapidly binding to the injured endothelium, thrombus formation was studied in CX3CR1GFP mice. CX3CR1GFP mice coexpress green fluorescent protein (GFP) with CX3CR1 so that their monocytes, monocyte-derived microparticles, macrophages, and dendritic cells (DCs) are all fluorescently labeled.27 Exogenous monocytes and monocyte-derived microparticles were previously shown to play a role in thrombus formation.13 The kinetics of thrombus formation was similar in CX3CR1GFP and wild-type mice (Figure 3A). Although macrophages and monocytes were detected in the cremaster tissue and circulating in veins, no GFP signal was detected at the site of thrombus formation during the first 3 minutes after a laser-induced injury (Figure 3B, supplemental Video 1). Three to 5 minutes after the injury, GFP-expressing cells were detected rolling on the thrombus and bound to it 25 minutes later (Figure 3B). Altogether, these results indicate that monocytes are not required for the formation of the platelet thrombus in arteries.
Kinetics of monocyte accumulation at the site of injury. (A) Kinetics of platelet accumulation in wild-type mice (32 thrombi, 3 mice) and in CX3CR1GFP mice (31 thrombi, 3 mice). (B) Representative images of a thrombus in CX3CR1GFP mice. GFP-monocyte (green) and platelet (red) accumulation was observed at the site of injury.
Kinetics of monocyte accumulation at the site of injury. (A) Kinetics of platelet accumulation in wild-type mice (32 thrombi, 3 mice) and in CX3CR1GFP mice (31 thrombi, 3 mice). (B) Representative images of a thrombus in CX3CR1GFP mice. GFP-monocyte (green) and platelet (red) accumulation was observed at the site of injury.
Neutrophils are the main population of granulocytes and constitute approximately 20% of total leukocytes circulating in the blood of mice. The 1A8 antibody is directed against Ly-6G, which is mainly expressed in circulation by neutrophils, although approximately 20% of circulating monocytes, identified as inflammatory monocytes, also expressed this marker (supplemental Figure 2A). After infusion of the 1A8 antibody into wild-type mice, a fluorescent signal was detected at the site of injury (Figure 4A). The signal appeared before any platelets could be detected and it increased over time (Figure 4B). To confirm that neutrophils were the subpopulation of leukocytes accumulating at the site of injury, experiments in CX3CR1GFP mice were performed. Neutrophils were detected by infusion of 1A8 antibody; monocytes, monocyte-derived microparticles, DCs, and macrophages were detected by the expression of GFP. Platelets were labeled by infusion of an anti-CD41 antibody; inflammatory monocytes expressed GFP and were labeled by 1A8. We observed that neutrophils, but neither monocytes nor inflammatory monocytes, accumulated at the site of injury in the seconds after the injury (Figure 4C, supplemental Video 2). Three to 5 minutes after the injury, inflammatory monocytes were detected rolling over the thrombus (Figure 4D). We conclude that neutrophils were the exclusive circulating cells that bound to the activated endothelium in the second after the arterial injury before platelets. Previous studies have shown that leukocytes participate in the activation of the blood coagulation cascade.2,28,29 After a laser-induced injury, thrombin is the main platelet agonist involved in thrombus formation.10,15,25 We confirmed that thrombus formation and fibrin generation were largely reduced in low TF mice (supplemental Figure 2B). PMNs assemble the FXII-driven contact pathway that initiates the intrinsic pathway of coagulation.30 We compared the kinetics of platelet accumulation and fibrin generation in wild-type and FXII−/− mice.16 Our results indicate that both thrombus formation and fibrin generation were similar in wild-type mice and FXII−/− mice (Figure 4E). These data were confirmed in wild-type mice infused with corn trypsin inhibitor (CTI; an inhibitor of activated FXII). CTI affected neither the kinetics of thrombus formation nor fibrin generation after laser-induced injury in wild-type mice (supplemental Figure 3). Altogether, these results indicated that after a laser-induced injury, TF was the main trigger leading to the generation of thrombin and the formation of a thrombus.
In vivo imaging of platelets, neutrophils, and monocytes during thrombus formation. (A-B) Representative images of (A) Ly-6G or (B) platelets and Ly-6G neutrophils at site of injury in wild-type mice (26 thrombi, 3 mice). (C) Thrombus formation in mice expressing CX3CR1GFP after infusion of CD41 and Ly-6G antibody. Monocytes (green), platelets (red), and neutrophils (blue) are visualized over time after laser injury. (D) Representative images showing an inflammatory monocyte rolling on a thrombus. The monocyte is depicted in white (composite of blue plus green plus red) or in light blue (composite of dark blue and green). Platelets are in red, neutrophils are in dark blue, monocytes, macrophages and DC are depicted in green. (E) Platelets and fibrin generation in wild-type mice and FXII−/− mice. Graphs represent medians of fibrin-integrated fluorescence intensity in wild-type mice (29 thrombi, 3 mice) and FXII−/− mice (30 thrombi, 3 mice) over time.
In vivo imaging of platelets, neutrophils, and monocytes during thrombus formation. (A-B) Representative images of (A) Ly-6G or (B) platelets and Ly-6G neutrophils at site of injury in wild-type mice (26 thrombi, 3 mice). (C) Thrombus formation in mice expressing CX3CR1GFP after infusion of CD41 and Ly-6G antibody. Monocytes (green), platelets (red), and neutrophils (blue) are visualized over time after laser injury. (D) Representative images showing an inflammatory monocyte rolling on a thrombus. The monocyte is depicted in white (composite of blue plus green plus red) or in light blue (composite of dark blue and green). Platelets are in red, neutrophils are in dark blue, monocytes, macrophages and DC are depicted in green. (E) Platelets and fibrin generation in wild-type mice and FXII−/− mice. Graphs represent medians of fibrin-integrated fluorescence intensity in wild-type mice (29 thrombi, 3 mice) and FXII−/− mice (30 thrombi, 3 mice) over time.
The presence of PMNs is required for the activation of coagulation by the TF pathway
To determine whether PMNs are required for activation of coagulation, we compared thrombus formation and fibrin generation before and after blocking of ICAM-1 to prevent neutrophil binding to activated endothelial cells. Thrombus formation and fibrin generation were both significantly reduced when the binding of neutrophils to the endothelium was inhibited (Figure 5). These results were confirmed in wild-type mice after a pretreatment with the GR-1 antibody. In such mice, 99% of circulating PMNs were depleted in comparison with wild-type mice (supplemental Figure 4A). After a laser-induced injury, thrombus formation and fibrin generation were significantly reduced (supplemental Figure 4B-C). Altogether, these results indicate that the interaction of PMNs with the injured endothelial wall is required for the activation of coagulation by the TF pathway.
Neutrophils activate the coagulation TF pathway leading to thrombus and fibrin formation. (A) Platelet accumulation at the site of injury in wild-type mice in absence or presence of ICAM-1–blocking antibody (32 thrombi, 3 mice for each condition). Graph represents medians of platelet-integrated fluorescence intensity in presence or absence of ICAM-1–blocking antibody over time. (B) Fibrin generation in wild-type mice in presence or absence of ICAM-1–blocking antibody. Graph represents medians of fibrin-integrated fluorescence intensity in WT mice in presence (28 thrombi in 3 mice) or absence (40 thrombi in 3 mice) of ICAM-1–blocking antibody over time.
Neutrophils activate the coagulation TF pathway leading to thrombus and fibrin formation. (A) Platelet accumulation at the site of injury in wild-type mice in absence or presence of ICAM-1–blocking antibody (32 thrombi, 3 mice for each condition). Graph represents medians of platelet-integrated fluorescence intensity in presence or absence of ICAM-1–blocking antibody over time. (B) Fibrin generation in wild-type mice in presence or absence of ICAM-1–blocking antibody. Graph represents medians of fibrin-integrated fluorescence intensity in WT mice in presence (28 thrombi in 3 mice) or absence (40 thrombi in 3 mice) of ICAM-1–blocking antibody over time.
PMNs are the main source of TF present at the site of injured vessel wall
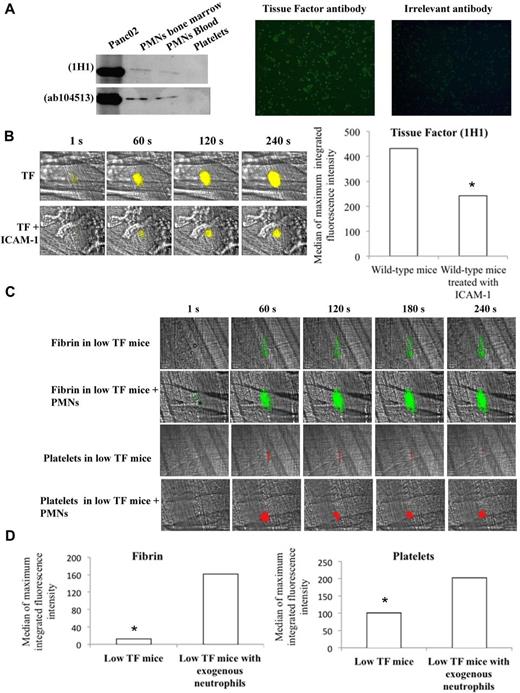
Previous studies have shown that in vitro neutrophils contain TF and may express it at the cell surface.3,16,31 In the present study, using 2 different anti-TF antibodies, a band corresponding to TF was observed by Western blot in PMNs purified from the bone marrow and from blood (Figure 6A left panel). The presence of TF in purified PMNs was confirmed in vitro by immunofluorescence only after permeabilization of the cells (Figure 6A right panel), indicating that naive PMNs do not express TF at the cell surface. In vivo, TF was detected at the site of injury in the blood circulation immediately after a laser-induced injury using 2 different antibodies directed against mouse TF (Figure 6B, supplemental Figure 5). When PMN binding was prevented by the use of ICAM-1–blocking antibody, the signal corresponding to TF was significantly reduced (Figure 6B, supplemental Figure 5), indicating that in vivo, after the interaction with endothelial cells, PMNs expressed TF at the cell surface. To determine whether the interaction of PMNs with the endothelial cells is required and sufficient for thrombus formation and fibrin generation, purified PMNs from wild-type mice were infused into low TF mice. After infusion of PMNs, fibrin generation and platelet accumulation were significantly increased after a laser-induced injury (Figure 6C-D). These results indicate that PMNs expressing TF are the main source of TF needed for platelet thrombus formation and fibrin generation after a laser-induced injury in vivo.
Neutrophils contain TF in vitro and expressed it in vivo at the site of laser-induced injury. (A) In vitro detection of TF in neutrophils; Western blot analysis of protein extracts from purified Panc02 mouse pancreatic cancer cells, bone marrow neutrophils, circulating neutrophils, and washed platelets with 2 anti–mouse TF antibodies (left panel). Immunofluorescence microscopy of neutrophils (PMNs) isolated from mice with an antibody directed against mouse TF with an Alexa 488–conjugated secondary antibody. Negative control was performed with an irrelevant antibody and the secondary antibody. Original magnification, ×200; n = 3 (right panel). (B) Representative pictures showing the visualization of TF in the bloodstream of wild-type mice at the site of laser-induced injury in presence or absence of ICAM-1–blocking antibody using the mouse anti-TF antibody 1H1. TF is shown in yellow. Graph represents the sum of the medians of TF-integrated fluorescence intensity in wild-type mice in presence (32 thrombi in 3 mice) or absence (31 thrombi in 3 mice) of ICAM-1–blocking antibody. (C) Fibrin generation and thrombus formation at the site of laser-dye injury in low TF mice before and after infusion of isolated neutrophils (PMNs). Fibrin is depicted in green; platelets are in red. (D) Graph depicts medians of fibrin and platelet-integrated fluorescence intensity in low TF mice (32 thrombi, 3 mice) and low TF mice after infusion of PMNs (31 thrombi, 3 mice).
Neutrophils contain TF in vitro and expressed it in vivo at the site of laser-induced injury. (A) In vitro detection of TF in neutrophils; Western blot analysis of protein extracts from purified Panc02 mouse pancreatic cancer cells, bone marrow neutrophils, circulating neutrophils, and washed platelets with 2 anti–mouse TF antibodies (left panel). Immunofluorescence microscopy of neutrophils (PMNs) isolated from mice with an antibody directed against mouse TF with an Alexa 488–conjugated secondary antibody. Negative control was performed with an irrelevant antibody and the secondary antibody. Original magnification, ×200; n = 3 (right panel). (B) Representative pictures showing the visualization of TF in the bloodstream of wild-type mice at the site of laser-induced injury in presence or absence of ICAM-1–blocking antibody using the mouse anti-TF antibody 1H1. TF is shown in yellow. Graph represents the sum of the medians of TF-integrated fluorescence intensity in wild-type mice in presence (32 thrombi in 3 mice) or absence (31 thrombi in 3 mice) of ICAM-1–blocking antibody. (C) Fibrin generation and thrombus formation at the site of laser-dye injury in low TF mice before and after infusion of isolated neutrophils (PMNs). Fibrin is depicted in green; platelets are in red. (D) Graph depicts medians of fibrin and platelet-integrated fluorescence intensity in low TF mice (32 thrombi, 3 mice) and low TF mice after infusion of PMNs (31 thrombi, 3 mice).
Discussion
Our results demonstrated that the interaction of neutrophils with the injured endothelial cells, through LFA-1/ICAM-1 interactions, is the first step leading to the generation of fibrin and thrombus formation in the laser-induced injury model of cremaster arterioles. Altogether, these data identify neutrophils as a key partner involved in thrombosis by providing TF before platelet accumulation. In addition to this new key role in hemostasis, blood neutrophils are crucial players in innate immunity, participating in antimicrobial machinery. This crosstalk between innate immunity and blood coagulation may represent an efficient strategy to prevent both infection and bleeding.
The traffic signals for neutrophil and monocyte localization during inflammation function in a 3-step process. This molecular model was described according to the observations that at sites of inflammation, leukocytes first roll on the vessel wall and then become firmly adhered at a single location on the vessel wall before the last-step diapedesis.32 Tethering brings leukocytes into proximity with chemoattractants that are present at sites of injury/inflammation. Chemoattractants activate integrins. Activated integrins can then interact with their ligands and induce the firm adhesion of leukocytes at the site of injury. The immediate adhesion of neutrophils to the vessel wall was mainly dependent on interactions between LFA-1 expressed by neutrophils and ICAM-1 expressed by activated endothelial cells. This interaction between an integrin and its ligand may occur spontaneously as is the case for the firm adhesion of platelets to immobilized fibrinogen. Indeed, different studies have shown that chemoattractants stimulate strong, integrin-mediated adhesion to bilayers containing ICAM-1 under static conditions but not in shear flow conditions.33 In addition, it is still unknown whether chemoattractants can act in the bloodstream, where they would be rapidly diluted and swept downstream by the blood flow.34 The selective and varied responses of PMNs and monocytes to injury can be explained by their receptivity to the distinct combinations of molecular signals such as LFA-1/ICAM-1 and P-selectin/PSGL-1. Our observations indicate that neutrophils, under arteriole flow conditions, stably attach to the vessel wall in the seconds after the injury. Different publications have shown that neutrophils have the ability to roll at 10-fold higher shear stress in vivo than in vitro.35,36 This may occur via the formation of slings, recently described as the essential mechanism involved in high-shear rolling of neutrophils.37 Lastly, it is possible that only a subpopulation of neutrophils that have, for instance, performed a reverse transmigration are able to adhere rapidly at the site of injury.
The use of real-time digital intravital microscopy has helped the comprehension of cellular mechanisms involved in thrombus formation. However, how the blood coagulation cascade is activated after an injury has remained unclear. Falati et al proposed that monocyte-derived microparticles that expose TF accumulate at the site of injury via interactions between P-selectin expressed on activated platelets and PSGL-1 expressed on monocyte-derived microparticles.13 However, these experiments were performed by infusion of exogenously isolated monocyte-derived microparticles and failed to show that such endogenous microparticles exist and indeed interact with a platelet thrombus in vivo. When we studied thrombus formation in CX3CR1GFP mice, we did not observe any signal corresponding to an accumulation of endogenous monocyte-derived microparticles at the site of injury. We concluded that the accumulation of monocyte-derived microparticles was not essential for thrombus formation and fibrin generation. This discrepancy may be explained by the fact that Falati et al used exogenous purified, labeled monocyte-derived microparticles.13 We concluded that neutrophils are the main source of blood-borne TF in the model of laser-induced injury. Infusion of neutrophils only partially restores thrombus formation in low TF mice. This may indicate that other sources of TF are involved after a laser-induced injury, including the vascular wall.15 Thus, after a laser-induced injury, neutrophils may represent the initial source of blood-borne TF needed to generate thrombin. Vascular TF could be involved for optimal thrombus growth. Additional studies are needed to discriminate the contribution of blood-borne versus vascular TF in this model of thrombosis.
A candidate identified as a possible activator of TF in vivo was the enzyme protein disulfide isomerase (PDI). Indeed, inhibition of PDI reduced fibrin generation and thrombus formation.38,39 However, PDI plays roles in platelet activation that may explain this result. For instance, PDI is needed to maintain the platelet integrin αIIbβ3 in the right configuration, leading to inside-out and outside-in signaling.40 PDI may also participate with neutrophils in TF activation. Indeed, PMNs may express inactive TF that could be activated by PDI secreted by activated endothelial cells and platelets.39 We observed that active TF is expressed at the surface of PMNs at the site of injury. According to our observations, neutrophils represent the main source of blood-borne TF in the laser model of thrombosis. However, the cellular source of blood-borne TF remains controversial.41 It is possible, as previously suggested, that TF was transferred from monocytes or monocyte-derived microparticles to PMNs.6
Massberg et al have recently shown that blood -activated neutrophils can inactivate TFPI via the release of proteases at the site of injury.2 In this model, the subendothelial matrix is exposed to blood leading to the formation of a platelet thrombus. Here, we demonstrate, using the laser model of injury, that neutrophil interaction with endothelial cells is required for thrombus formation. This interaction may not occur when the subendothelial matrix is exposed to the blood circulation. Neutrophils may thus not be able to support the first steps of thrombus formation in this model. However, their presence was described to be important for formation of an occlusive thrombus.3 In the present study, we focused on the initial steps leading to thrombus formation after a laser-induced injury. We observed a rapid and a constant recruitment of neutrophils. Thus, interactions of neutrophils with platelets may occur independently of the model of injury. This interaction may be involved for the optimal growth of a thrombus in cardiovascular diseases, such as myocardial infarction, as well as in infectious disease. Alternatively, in the ferric chloride model, neutrophils may also bind to the endothelial cells surrounding the site of injury. Hypothetically, this may lead to the formation of multiple microthrombi, similar to the one observed after a laser-induced injury, and will ultimately induce the occlusion of the artery observed in this model of injury.
Apart from the TF pathway, activation of coagulation may also occur through the FXII-dependent contact pathway.16 Using FXII−/− mice, we observed that the extrinsic pathway was the exclusive pathway involved in the first steps leading to the initiation phase of coagulation after a dye-laser injury. Previous publications have reported that neutrophils may serve as a matrix for the activation of the contact phase.7 It is, therefore, possible that in models of thrombus formation that are dependent on FXII activation, neutrophils may also play a critical role. In our model, which is strictly dependent on TF activation, PMNs played a major role as the unique source of TF. In addition, PMNs may also inactivate TFPI. It is also possible that neutrophils, by producing NETs,42 could directly activate TF. Further studies are needed to identify the exact molecular mechanisms involved in TF activation and TFPI inactivation by PMNs.
Although neutrophils seem to be involved in both arterial and venous injuries, their role may not be the same in different vessel beds. Indeed, in a mouse model of DVT, von Brühl et al demonstrate by intravital 2-photon microscopy that blood monocytes and neutrophils crawling along and adhering to the venous endothelium provide the initiating stimulus for DVT development.3 The authors show that thrombus-resident neutrophils are indispensable for subsequent DVT propagation by binding FXII and by supporting its activation through the release of NETs. In this model of venous thrombosis, platelets promote leukocyte recruitment and stimulating neutrophil-dependent coagulation. In our model of arteriole thrombosis, neutrophils participate in the activation of the extrinsic pathway of coagulation via TF, independent of FXII and the presence of platelets and monocytes at the site of injury. Inhibiting PMN from interacting with the endothelium at the site of injury prevents both accumulation of platelets and activation of the blood coagulation cascade. Our results identified that the interaction of neutrophils with the injured endothelial cells, through LFA-1/ICAM-1 interactions, is the first step leading to the generation of fibrin and thrombus formation.
Several vascular risk factors are associated with proinflammatory alterations, including leukocyte activation, and predispose cerebral vasculature to thrombogenesis on inflammatory stimulation.4 For example, accumulation of inflammatory cells within the vascular wall starts early during atherogenesis. During later disease stages, their activation can lead to plaque rupture and thrombus formation, increasing the risk of stroke and acute coronary syndromes. Moreover, the association of an acute inflammatory reaction with unstable angina has been previously established and a variety of experimental studies suggested that neutrophil-induced endothelial dysfunction occurred near the site of coronary obstruction.5,43 Our model of injury is strictly dependent on endothelial activation.26 We now show that participation of neutrophils is essential for thrombus formation. This may indicate that the laser model mimics what may occur after thrombus formation induced by—or in relation with—inflammatory events, such as after lung inflammation,44 involvement of Shiga toxin thrombotic mechanisms in hemolytic uremic syndrome,45 or thrombus formation in cerebral microvessels involved in sickle cell disease.46 Our results indicate that preventing the binding of PMNs to the vessel wall could potentially represent a therapeutic strategy to prevent arterial thrombosis and inflammation. Targeting neutrophil adhesion interacting with endothelial cells may thus efficiently reduce the risks of a stroke and prevent acute coronary syndromes or acute cerebral ischemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to Stephane Robert (Inserm UMR-S1076) for technical assistance. They also thank Dr Markus Riederer (Actelion Switzerland) for critical scientific review of the work, and Ben Atkinson (Intelligent Imaging Innovation) for technical support of the intravital system.
This work was supported by the Fondation pour la Recherche Médicale (FRM) Association (C.D.), and by grants from institutional funding from Inserm, the Aix-Marseille University, the Agence Nationale pour la Recherche (ANR; grant ANR-09-JCJC-0053; C.D.), and the Agence pour la Recherche sur le Cancer (ARC) Association (C.D.).
Authorship
Contribution: R.D., S.M., C.F., and R.B. contributed to the design and performance of the research, analysis of data, and writing of the manuscript; and G.M.T., N.M., T.R., F.D.-G., C.D., and L.P.-D. contributed to the experimental design, analysis of data, and writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christophe Dubois, Faculty of Pharmacy, Inserm UMR-S1076, 27 Blvd Jean Moulin, 13385 Marseille, France; e-mail: christophe.dubois@univ-amu.fr.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal