Abstract
Dendritic cells (DCs) can capture extracellular antigens and load resultant peptides on to MHC class I molecules, a process termed cross presentation. The mechanisms of cross presentation remain incompletely understood, particularly in primary human DCs. One unknown is the extent to which antigen delivery to distinct endocytic compartments determines cross presentation efficiency, possibly by influencing antigen egress to the cytosol. We addressed the problem directly and quantitatively by comparing the cross presentation of identical antigens conjugated with antibodies against different DC receptors that are targeted to early or late endosomes at distinct efficiencies. In human BDCA1+ and monocyte-derived DCs, CD40 and mannose receptor targeted antibody conjugates to early endosomes, whereas DEC205 targeted antigen primarily to late compartments. Surprisingly, the receptor least efficient at internalization, CD40, was the most efficient at cross presentation. This did not reflect DC activation by CD40, but rather its relatively poor uptake or intra-endosomal degradation compared with mannose receptor or DEC205. Thus, although both early and late endosomes appear to support cross presentation in human DCs, internalization efficiency, especially to late compartments, may be a negative predictor of activity when selecting receptors for vaccine development.
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells that process proteins and load resultant peptides on to MHC molecules. On activation, DCs migrate to secondary lymphoid organs and present antigen to their cognate T cells, for the induction of adaptive immune responses.1 Because DCs are essential for the initiation of adaptive immune responses, they are attractive targets for enhancing prophylactic and therapeutic vaccination strategies2-4 and have been shown to activate antitumor immune responses in murine cancer models.5,6
In human cancer, clinical evidence is now accumulating to suggest that the induction or activation of CD8+ T-cell immunity can contribute to the arrest of tumor growth and patient survival.7 In principle, targeting tumor antigens to DCs may enhance protective CD8+ T-cell responses because of the ability of DCs to cross present exogenous antigens.8 In cross presentation, exogenous proteins are endocytosed, processed, and loaded on to MHC class I molecules for presentation to CD8+ T cells. The efficiency of cross presentation can be improved greater than 100-fold when receptors found on the surface of DCs are targeted specifically.6
Multiple DC populations exhibit some capacity for cross presentation in vitro, but certain subpopulations (CD8+ DCs in the mouse, BDCA3+ DCs in humans) are thought to be particularly adept in vivo.8 DCs exhibit a variety of surface receptors that can internalize antigen for cross presentation, with some being subpopulation specific. Pioneering studies from Bozzacco et al and Idoyaga et al have shown cross presentation using the C-type lectin receptor DEC205, which is expressed by CD8+ mouse DCs as well as by multiple human DC subsets.9,10 Another lectin receptor, CLEC9A/DNGR-1, is expressed by mouse CD8+ DCs and human BDCA3+ DCs and also mediates cross presentation.5 Indeed, a variety of receptors expressed by human or mouse DCs (DCIR, Langerin, mannose receptor [MR]), DC-SIGN, CLEC12A) enable cross presentation.10-15 Which of these receptors is most efficient and why remains poorly understood, particularly in human DCs.
A few recent studies have begun to address the relative roles of early endosomes and late endosomes/lysosomes in cross presentation. It was suggested for mouse DCs that cross presentation of soluble ovalbumin internalized by MR, presumably targeted to early endosomes, is more efficient than cross presentation after fluid phase uptake, which also delivers an unspecified portion of soluble ovalbumin to late endosomes.16-18 Early endosomes also may host the cross presentation of liposome-encapsulated hen egg lysozyme, but in a fashion independent of the proteasomal pathway.19,20 Experiments where an antigen was coupled to DC-SIGN antibodies also suggested that early endosomal targeting may facilitate cross presentation by human DC-SIGN transgenic mouse DCs.13 In all of these studies, however, there was limited quantitation or characterization of antigen accumulation and fate, and direct and effective comparisons were not made between the various uptake routes. Where the effects of targeting different receptors was compared, endosomal targeting was not well studied, and the conjugates were poorly characterized for the possibility of aggregates,21,22 an important consideration if any of these platforms are to be transferred to the clinic for use in humans. Aggregates also tend to be transferred to lysosomes and may have altered degradation properties.23,24 Finally, suggestions that early endosomal targeting is optimal appear inconsistent with more extensive investigations of DEC205, which is targeted primarily to late endocytic compartments25,26 but appears to mediate the efficient cross presentation of proteins coupled to or fused to anti-DEC205 antibodies.6,9,12
To establish directly and quantitatively the endosomal requirements for efficient cross presentation using a clinically relevant and scalable platform, we used elongated peptide antigens conjugated to monoclonal antibodies against 3 receptors that traffic to distinct cellular compartments: CD40, MR, and DEC205. Interestingly, we found an inverse relationship between internalization or antigen degradation and cross presentation: antigens destined for more degradative late endosomes were poorly cross presented relative to the same antigens targeted to early endosomes, an effect that was independent of the amount of antigen internalized.
Methods
Cell isolation and culture
This study was approved by the Genentech Institutional Review Board. Our procedures for the isolation of DCs and T cells from blood have been described previously.27 Healthy donors were leukapheresed, and enriched populations of lymphocytes and monocytes were obtained by counterflow elutriation. BDCA1+ DCs were isolated from elutriated monocytes with the use of the CD1c DC isolation kit and AutoMACS technology (Miltenyi Biotec). Cells were cultured overnight in R10 (RPMI 1640 + Glutamax with 10% FCS, 100 U/mL penicillin + 100 μg/mL streptomycin, 10mM HEPES; Gibco/Invitrogen), and 2 ng/mL GM-CSF (PeproTech). Monocyte-derived DCs (MoDCs) were derived in 5-7 days from CD14+ monocytes cultured in R10 with 100 ng/mL GM-CSF (PeproTech) and 6.5 ng/mL IL-4 (R&D Systems). To mature DCs, DCs were exposed to 0.2 μg/mL lipopolysaccharide (LPS; Sigma-Aldrich) overnight. For autologous CD8+ T-cell assays with MoDCs, elutriated HLA-A*0201+ monocytes were frozen in anticipation of donor recall. CD8+ T cells were isolated from HLA-A*0201+ elutriated lymphocytes with the use of the CD8+ T-cell isolation kit (Miltenyi Biotec). For CD4 T-cell assays that used an NY-ESO peptide-specific human CD4 T-cell clone (CCNE-1 415; obtained from Cassian Yee, Fred Hutchinson Cancer Research Center, Seattle, WA), HLA-DPB1*0401 DCs were obtained as outlined earlier in this paragraph.
Antibodies
Anti-DEC205 (3G9; Kd, 0.3nM) and anti-MR (B11; Kd, 0.7nM) used in our studies were obtained in collaboration with Celldex Therapeutics, and the anti-CD40 (S2C6; Kd, 0.12nM) was obtained in collaboration with Seattle Genetics. Binding affinities were determined by flow cytometry.
Antibodies used for flow cytometry/immunofluorescence were as follows: anti-CD3 (SK7), CD4 (Leu-3a/SK3), CD8 (SK1), CD8 (RPA-T8), CD11c (B-ly6), CD14 (MΦP9), CD19 (Leu-12), CD40 (5C3), CD71 (M-A712), CD83 (HB15e), CD86 (FUN-1), CD206 (19.2), DEC205 (MG38), HLA-DR (TU36), IFNγ (B27), IL-2 (MQ1-17H12), Lamp1 (H4A3), and TNFα (6401.1111; BD Biosciences); anti–HLA-DR (L243; Biolegend); anti–HLA-ABC (W6/32; eBioscience); anti-CD1a (NA1/34; Dako); anti-EEA1 (Cell Signaling); and secondary reagents (streptavidin Alexa 555/647, anti–rabbit Alexa 546, anti-FITC Alexa 488; Invitrogen/Molecular Probes).
Synthesis of antibody-peptide conjugates
Antibodies in PBS buffer with 50mM potassium phosphate (pH 7.5) were reacted with a 10- to 15-fold molar excess of N-succinimidyl S-acetylthioacetate (Pierce) for 4-6 hours at 20°C. Excess reagents were removed by dialysis in PBS, resulting in antibodies with 3-6 blocked thiol groups. Blocking groups were then removed with 20mM hydroxylamine (Sigma-Aldrich), and peptides containing an N-terminal maleimide (Elim Biopharmaceuticals) were added to ∼ 5- to 6-fold molar excess over antibody and allowed to react with the new thiol groups for 2-4 hours at 20°C. Excess peptide and aggregated antibodies were removed by gel filtration in PBS. Electrospray mass spectrometry was used to estimate the average number of peptides per antibody. The peptides conjugated to antibodies were (immunodominant epitopes are italicized and sequence position indicated): LTKGILGFVFTLTVPSER (influenza M1 58-66), QAGILARNLVPMVATVQGQNL (CMV pp65 495-503), or QQLSLLMWITQAFLPVFLAQPPSGQRR (NY-ESO 157-170).
Immunofluorescence
After culture or accumulation with 1 μg/mL anti-CD40 (S2C6), anti-MR (B11), or anti-DEC205 (3G9) antibodies covalently conjugated to Alexa 488 (Alexa 488 monoclonal antibody kit; Invitrogen), BDCA1+ DCs or MoDCs were washed and spotted on Alcian blue–coated coverslips for 10-15 minutes at room temperature. Cells were fixed in 4% paraformaldehyde (Electron Microscopy Source) for 15 minutes, permeabilized in 0.05% saponin (Sigma-Aldrich), and counterstained. When biotinylated antibodies were used, endogenous biotin was blocked first with the use of an excess of unlabeled streptavidin and biotin (Endogenous Biotin Blocking Kit; Invitrogen). After labeling, coverslips were mounted onto glass slides with the use of Prolong Gold with 4′,6′-diamidine-2-phenylindole (Invitrogen/Molecular Probes). Images were acquired on a Leica SP5 confocal microscope at room temperature (Leica Microsystems), using a 100× oil objective (NA, 1.47) with zoom 7, and Leica's LAS Version 2.6.0.7266 imaging software (Leica Microsystems).
Antibody accumulation experiments
Cells were continuously incubated with 1 μg/mL anti-CD40 (S2C6), anti-MR (B11), or anti-DEC205 (3G9) Alexa 488 antibodies for indicated times, in the presence or absence of the serine/cysteine protease inhibitor leupeptin (5mM; Roche) and the acidophilic weak base ammonium chloride (NH4Cl; 10mM). After incubations, cells were stained for CD14 and maturation markers, fixed, acquired with a BD FACSCanto II, and analyzed for Alexa 488 signal with the use of FlowJo Version 9.4.11 software (TreeStar). For direct receptor comparisons, mean fluorescence intensity (MFI) was normalized for surface antibody signal (4°C) and the number of fluors per antibody.
Antigen presentation assays
MHC class I.
HLA-A*0201 DCs were incubated for 4-6 hours in the presence of antibody-peptide conjugates (0.1-10 μg/mL) or other reagents of interest (peptide control 2.5-250 ng/mL; Proimmune; epoxomicin, 0.1μM; Calbiochem; DMSO, 0.1μM; Sigma-Aldrich). After antigen uptake, DCs were washed extensively to remove free antibody, peptide, or inhibitor and were cocultured with CD8+ T cells labeled with carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) for 8-10 days at a 1:30 ratio of DCs to T cells in the presence of 20 U/mL IL-2 (Roche) and 200 ng/mL LPS (Sigma-Aldrich). After coculture, influenza M1 or CMV pp65 peptide-specific CD8+ T cells were labeled with a corresponding PE-pentamer (ProImmune), acquired with the use of a BD FACSCanto II, and gated on CD3+ CD8+ (CD14/CD4/CD19−) cells. CFSElo/pentamerhi cells were analyzed with FlowJo Version 9.4.11 (TreeStar).
MHC class II.
HLA-DPB1*0401 DCs were incubated for 1.5 hours in the presence of antibody conjugates (0.1-10 μg/mL) or other reagents of interest (peptide control 2.5-25 μg/mL; Elim Biopharmaceuticals; SEB 1 μg/mL; Sigma-Aldrich). After antigen uptake, DCs were washed and cocultured with an NY-ESO peptide-specific human CD4 T-cell clone for 7-9 hours at DC to T-cell ratios of 1:1, 1:3, and 1:9. Two hours into the coculture, brefeldin A (eBioscience) was added to prevent cytokine secretion. After coculture, cells were stained for surface markers, fixed, permeabilized, and labeled for intracellular IFNγ, IL-2, and TNFα. Cells were acquired with a BD FACSCanto II, and gated on CD3+CD4+CD1c− cells, followed by FlowJo Version 9.4.11 (TreeStar) analysis.
Pulse-chase experiments
Alexa 488–conjugated antibodies of interest were pulsed with cells at 37°C or 4°C for 1 hour at 4 μg/mL, in the presence or absence of 5mM leupeptin and 10mM NH4Cl. Cells were washed and chased for the indicated times, in the presence or absence of leupeptin and NH4Cl. Cells were counterstained with CD14 and maturation markers, washed, and fixed. Cells were acquired with a BD FACSCanto II and analyzed for Alexa 488 signal with the use of FlowJo Version 9.4.11 software (TreeStar).
Results
Antibodies are targeted to distinct cellular compartments
To compare quantitatively the ability of different endosomal compartments to facilitate cross presentation by DCs, we selected 3 receptors suspected to have distinct intracellular destinations or rates of internalization: MR, CD40, and DEC205. We first confirmed their surface expression on primary human BDCA1+ DCs and MoDCs (Figure 1A-C). With the exception of MR on BDCA1+ DCs, both CD40 and DEC205 were expressed at variably high levels by both immature and mature BDCA1+ DCs and MoDCs. MR expression was low but detectable on immature BDCA1+ DCs, but this amount further decreased on maturation. As expected, maturation enhanced CD40 and DEC205 expression in both DC types. When we compared relative receptor expression levels, both DC types expressed significant amounts of each receptor before and after maturation by LPS, except for MR whose expression was low in immature BDCA1+ DCs (Figure 1D). All antibodies exhibited subnanomolar affinity (anti-CD40, 0.12nM; anti-DEC205, 0.3nM; anti-MR, 0.7nM) and were used at saturation.
Human BDCA1+ DCs and MoDCs express MR, CD40, and DEC205. Donor-matched BDCA1+ DCs or MoDCs were washed and incubated with (A) anti-MR, (B) anti-CD40, or (C) anti-DEC205 antibodies for 25 minutes at 4°C. DCs were washed, fixed, and analyzed by flow cytometry. Histograms show 1 representative of 10 independent donors. (D) BDCA1+ DCs (closed symbols) and MoDCs (open symbols) were incubated with Alexa 488–conjugated anti-MR, CD40, or DEC205 for 25 minutes at 4°C, washed, and analyzed by flow cytometry. MFI was normalized for the number of fluorophores per antibody, to directly compare surface levels between receptors. The graph shows data from ≥ 3 independent donors with the mean MFI depicted.
Human BDCA1+ DCs and MoDCs express MR, CD40, and DEC205. Donor-matched BDCA1+ DCs or MoDCs were washed and incubated with (A) anti-MR, (B) anti-CD40, or (C) anti-DEC205 antibodies for 25 minutes at 4°C. DCs were washed, fixed, and analyzed by flow cytometry. Histograms show 1 representative of 10 independent donors. (D) BDCA1+ DCs (closed symbols) and MoDCs (open symbols) were incubated with Alexa 488–conjugated anti-MR, CD40, or DEC205 for 25 minutes at 4°C, washed, and analyzed by flow cytometry. MFI was normalized for the number of fluorophores per antibody, to directly compare surface levels between receptors. The graph shows data from ≥ 3 independent donors with the mean MFI depicted.
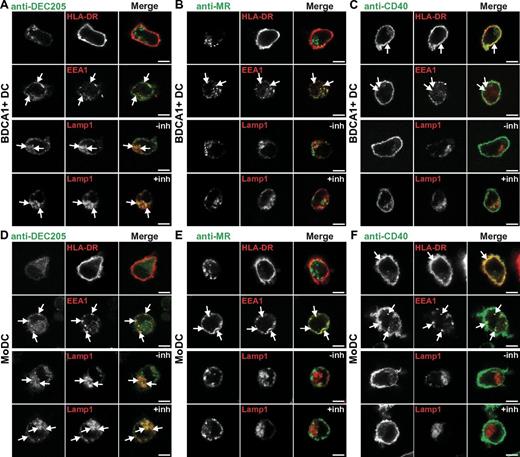
We next characterized the ability of each receptor to be internalized and its subsequent subcellular localization in both BDCA1+ DCs and MoDCs. As expected from previous studies, anti-DEC205 antibodies accumulated largely in late endosomes. Little was found on the surface of BDCA1+ DCs and MoDCs (Figure 2A,D first row), and a small amount appeared to be associated with early endosomes as marked by EEA1 (Figure 2A,D second row). The bulk of anti-DEC205 antibody overlapped with the late endosomal marker Lamp1 (Figure 2A,D third row), and the signal was enhanced in cells treated with the protease inhibitor leupeptin together with the acidophilic weak base NH4Cl (inh), indicating that antibody degradation was occurring in this compartment (Lamp1; Figure 2A,D fourth row). Therefore, in untreated cells the amount of anti-DEC205 delivery to late compartments was underestimated.
Targeting to DEC205 results in late endosomal localization, and CD40 and MR antibodies are largely excluded from late endosomes. (A-C) BDCA1+ DCs or (D-E) MoDCs were continuously incubated with 1 μg/mL fluorescently labeled (A,D) anti-DEC205, (B,E) anti-MR, or (C,F) anti-CD40 antibodies for 6 hours at 37°C. In parallel, DCs were incubated with antibodies in the presence of protease and acidification inhibitors leupeptin and NH4Cl (inh). DCs were seeded on coverslips and fixed, followed by staining for the cell surface (HLA-DR) and permeabilization and labeling of early endosomes (EEA1) or late endosomes (Lamp1). Arrows indicate areas of overlap. Images were acquired on a Leica SP5 confocal microscope, 100× oil objective (NA, 1.47), zoom 7. All immunofluorescence experiments were repeated in ≥ 5 independent donors. Scale bar is 5 μm.
Targeting to DEC205 results in late endosomal localization, and CD40 and MR antibodies are largely excluded from late endosomes. (A-C) BDCA1+ DCs or (D-E) MoDCs were continuously incubated with 1 μg/mL fluorescently labeled (A,D) anti-DEC205, (B,E) anti-MR, or (C,F) anti-CD40 antibodies for 6 hours at 37°C. In parallel, DCs were incubated with antibodies in the presence of protease and acidification inhibitors leupeptin and NH4Cl (inh). DCs were seeded on coverslips and fixed, followed by staining for the cell surface (HLA-DR) and permeabilization and labeling of early endosomes (EEA1) or late endosomes (Lamp1). Arrows indicate areas of overlap. Images were acquired on a Leica SP5 confocal microscope, 100× oil objective (NA, 1.47), zoom 7. All immunofluorescence experiments were repeated in ≥ 5 independent donors. Scale bar is 5 μm.
When MR antibody was incubated continuously with BDCA1+ DCs and MoDCs, it was mainly found intracellularly with relatively little on the cell surface (Figure 2B,E first row). Anti-MR was found in early endosomes as indicated by the partial overlap with EEA1 (Figure 2B,E second row) and minimal overlap with Lamp1 (Figure 2B,E third row). This pattern was maintained even in the presence of protease and acidification inhibitors (Figure 2B,E fourth row), suggesting that anti-MR antibodies did not reach late compartments as significantly as anti-DEC205 and did not escape detection because of degradation.
After continuous incubation in BDCA1+ DCs and MoDCs, anti-CD40 antibody was largely found on the surface (Figure 2C,F first row), in early endosomes (EEA1; Figure 2C,F second row), and minimally in late endosomes (Lamp1; Figure 2C,F third row). This pattern was similar in the presence of protease and acidification inhibitors (Figure 2C,F fourth row). Thus, like MR, anti-CD40 internalized to early endosomes but appeared to do so much less efficiently. Similar results were obtained for all antibodies at earlier and later time points of internalization (data not shown), and the extent of overlap was quantified (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
The patterns observed by antibody internalization were confirmed by indirect immunofluorescence detection of the receptors themselves; CD40 was mainly found on the surface, whereas MR and DEC205 were mainly found intracellularly, in early and in late endosomes, respectively (data not shown). In sum, we have characterized the targeting and localization of antibodies against 3 receptors in 2 different human DC types: DEC205 in late endosomes, MR in early endosomes, and CD40 on the surface and in early endosomes.
CD40 accumulates the least efficiently of the 3 receptors
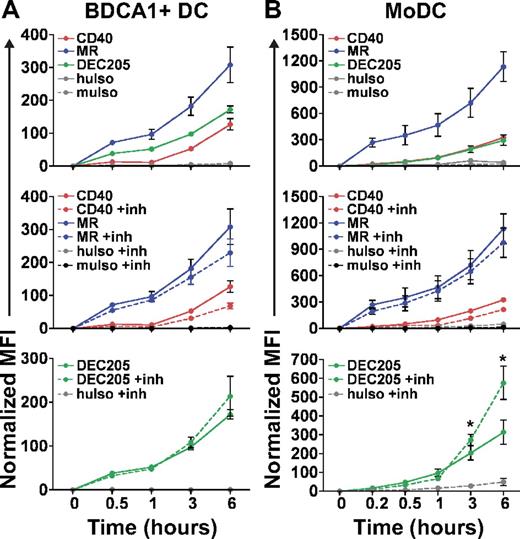
To quantify the ability of DEC205, MR, and CD40 to accumulate antigen, we determined the time course of continuous antireceptor antibody uptake by flow cytometry. As shown in Figure 3 for BDCA1+ DCs (panel A) and MoDCs (panel B), MR antibody was more effectively accumulated than either CD40 or DEC205 antibodies (Figure 3A-B top panels). Inhibiting proteolysis by the addition of protease and acidification inhibitors (inh) did not markedly affect the accumulation of either MR or CD40 antibodies but did significantly enhance the amount of cell-associated DEC205 antibody, particularly in MoDCs (Figure 3B bottom panel; P < .05). This finding is consistent with the observations that MoDCs exhibit higher levels of lysosomal enzymes than BDCA1+ DCs28,29 and that DEC205 antibodies are delivered predominantly to protease-containing late endosomes. In all cases, isotype controls were minimally internalized (muIso corresponds to anti-CD40; huIso corresponds to anti-MR and anti-DEC205). Taken together, these data indicate that MR and DEC205 antibodies are internalized by both DC types to a greater extent than CD40 antibodies.
Antibodies to DEC205 and MR are accumulated more efficiently by DCs than anti-CD40. (A) BDCA1+ DCs or (B) MoDCs were continuously incubated with 1 μg/mL fluorescently labeled anti-MR, anti-CD40, or anti-DEC205 for the indicated times, washed, counterstained, and fixed (top panels). Middle and bottom panels show data from the top panel, together with matched samples treated with leupeptin and NH4Cl (inh) during the continuous incubation. MuIso is the isotype for anti-CD40, and huIso is the isotype for anti-MR and anti-DEC205. Graphs depict the mean ± SEM normalized MFI from ≥ 3 independent donors. Normalized MFI indicates amount of accumulation; MFI was calculated by removing the contribution of surface fluorescence (4°C control) and normalizing the MFI for the number of fluorophores per antibody. Differences in antibody accumulation in the absence or presence of inhibitors were assessed with the paired t test, and statistically significant differences are depicted, *P < .05.
Antibodies to DEC205 and MR are accumulated more efficiently by DCs than anti-CD40. (A) BDCA1+ DCs or (B) MoDCs were continuously incubated with 1 μg/mL fluorescently labeled anti-MR, anti-CD40, or anti-DEC205 for the indicated times, washed, counterstained, and fixed (top panels). Middle and bottom panels show data from the top panel, together with matched samples treated with leupeptin and NH4Cl (inh) during the continuous incubation. MuIso is the isotype for anti-CD40, and huIso is the isotype for anti-MR and anti-DEC205. Graphs depict the mean ± SEM normalized MFI from ≥ 3 independent donors. Normalized MFI indicates amount of accumulation; MFI was calculated by removing the contribution of surface fluorescence (4°C control) and normalizing the MFI for the number of fluorophores per antibody. Differences in antibody accumulation in the absence or presence of inhibitors were assessed with the paired t test, and statistically significant differences are depicted, *P < .05.
Targeting CD40 and MR is more efficient for cross presentation than targeting DEC205
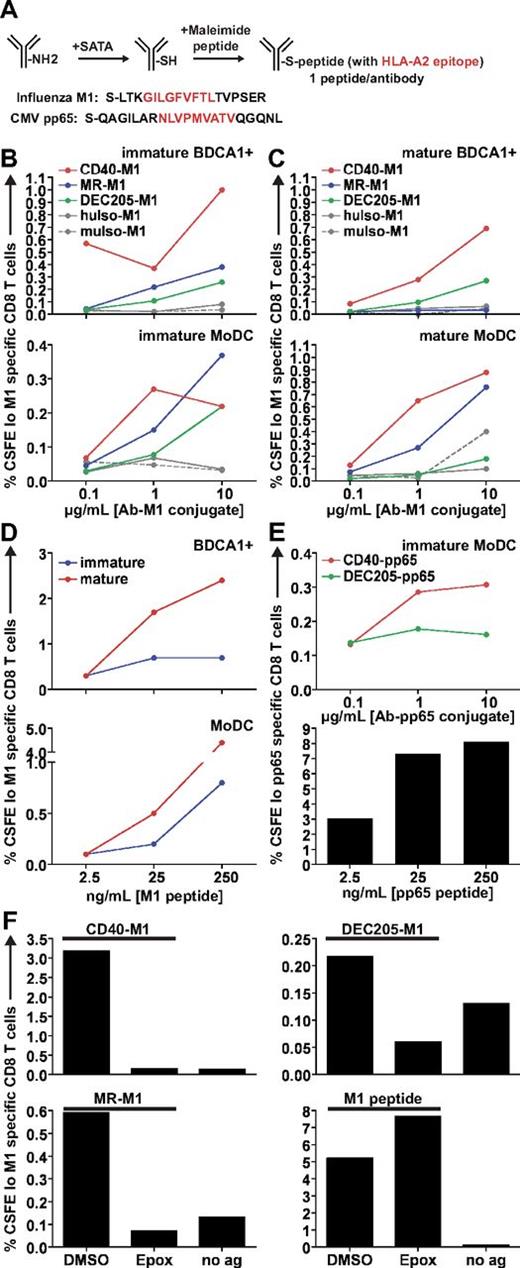
Having characterized the internalization and intracellular destinations of antibodies to CD40, DEC205, and MR, we next compared their relative abilities to mediate cross presentation. For this purpose, each high-affinity antibody was chemically coupled to extended peptides containing either of 2 immunodominant HLA-A*0201 epitopes, influenza M1 (58-66) or CMV pp65, (495-503) with the use of the strategy outlined in Figure 4A. Both epitopes (Figure 4A red residues) were extended by several residues of naturally occurring peptide sequence on the amino- and carboxy-terminal ends (Figure 4A black residues). We determined that the antibodies were conjugated to similar numbers of peptides per molecule by mass spectrometry and remained > 95% monomeric by gel filtration chromatography (data not shown).
Targeting CD40 and MR leads to superior cross presentation compared with targeting DEC205. (A) Schematic for the generation of antibody-peptide conjugates. On average, our peptide conjugates had 1 peptide per antibody. (B) Matched immature or (C) mature HLA-A*0201+ DCs were incubated with various concentrations of anti–CD40-M1, anti–MR-M1, or anti–DEC205-M1 for 4-6 hours, washed, and cocultured with autologous CFSE-labeled CD8+ T cells for 8-10 days in the presence of LPS and IL-2. “Immature” or “mature” refers to the activation state of the DCs before antigen uptake and coculture. MuIso-M1 is the isotype for anti–CD40-M1, and huIso-M1 is the isotype for anti–MR-M1 and anti–DEC205-M1. Graphs depict frequencies of total CFSElo and Influenza M1 (58-66) specific CD8+ T cells. One representative experiment of 6 to 8 independent donors (immature samples) or 3 to 4 independent donors (mature samples) is shown. Targeting via 1 μg/mL of anti-CD40 and MR was judged superior to targeting via anti-DEC205 across 6 to 8 independent donors with the use of a paired t test for immature DCs (CD40 vs DEC205: MoDC, P = .0270, BDCA1+ DC, P = .0337; MR vs DEC205: MoDC, P = .0264; BDCA1+ DC was not statistically significant possibly because of variable surface MR expression). (D) Influenza M1 (58-66) nonextended peptide control for antigen presentation assay. (E top panel) As in panel B, DCs were incubated with anti–CD40-pp65 or anti–DEC205-pp65 conjugates. (Bottom panel) CMV pp65 (495-503) nonextended peptide control. (F) DCs were incubated with 1 μg/mL antibody-M1 conjugates or 25 ng/mL M1 peptide with or without 0.1μM epoxomicin or DMSO for 4-6 hours, followed by washing and coculture with autologous CFSE-labeled CD8+ T cells. “No ag” shows the background when DCs and CD8+ T cells are cocultured in the absence of antigen. Graphs depict frequencies of total CFSElo and influenza M1 (58-66) specific CD8+ T cells. One representative experiment from ≥ 3 independent donors is shown.
Targeting CD40 and MR leads to superior cross presentation compared with targeting DEC205. (A) Schematic for the generation of antibody-peptide conjugates. On average, our peptide conjugates had 1 peptide per antibody. (B) Matched immature or (C) mature HLA-A*0201+ DCs were incubated with various concentrations of anti–CD40-M1, anti–MR-M1, or anti–DEC205-M1 for 4-6 hours, washed, and cocultured with autologous CFSE-labeled CD8+ T cells for 8-10 days in the presence of LPS and IL-2. “Immature” or “mature” refers to the activation state of the DCs before antigen uptake and coculture. MuIso-M1 is the isotype for anti–CD40-M1, and huIso-M1 is the isotype for anti–MR-M1 and anti–DEC205-M1. Graphs depict frequencies of total CFSElo and Influenza M1 (58-66) specific CD8+ T cells. One representative experiment of 6 to 8 independent donors (immature samples) or 3 to 4 independent donors (mature samples) is shown. Targeting via 1 μg/mL of anti-CD40 and MR was judged superior to targeting via anti-DEC205 across 6 to 8 independent donors with the use of a paired t test for immature DCs (CD40 vs DEC205: MoDC, P = .0270, BDCA1+ DC, P = .0337; MR vs DEC205: MoDC, P = .0264; BDCA1+ DC was not statistically significant possibly because of variable surface MR expression). (D) Influenza M1 (58-66) nonextended peptide control for antigen presentation assay. (E top panel) As in panel B, DCs were incubated with anti–CD40-pp65 or anti–DEC205-pp65 conjugates. (Bottom panel) CMV pp65 (495-503) nonextended peptide control. (F) DCs were incubated with 1 μg/mL antibody-M1 conjugates or 25 ng/mL M1 peptide with or without 0.1μM epoxomicin or DMSO for 4-6 hours, followed by washing and coculture with autologous CFSE-labeled CD8+ T cells. “No ag” shows the background when DCs and CD8+ T cells are cocultured in the absence of antigen. Graphs depict frequencies of total CFSElo and influenza M1 (58-66) specific CD8+ T cells. One representative experiment from ≥ 3 independent donors is shown.
Using these antibody conjugates, we performed recall cross presentation assays with human HLA-A*0201+ DCs and autologous CD8+ T cells from donors with detectable memory responses against influenza or CMV (supplemental Figure 2A). The percentage of total CD8+ T cells that were dividing and M1 or pp65 specific was assessed by CFSE dilution and pentamer staining. In immature BDCA1+ DCs and MoDCs fed with M1 peptide–coupled antibodies, targeting antibodies to the early endosomal receptors CD40 and MR was generally superior for cross presentation compared with DEC205 (Figure 4B; supplemental Figure 2B), which, surprisingly, was the most inefficient for cross presentation. Interestingly, the receptor that was least efficient in accumulating antigen, CD40, was the most efficient for cross presentation, indicating that intracellular antigen accumulation was not the rate-limiting step in determining cross presentation efficiency.
We also assessed the ability of mature DCs to cross present receptor-targeted antigen, because matured mouse DCs are able to present antigens captured via receptor-mediated endocytosis.26 We found that mature BDCA1+ DCs and MoDCs were able to induce efficient cross presentation via CD40, and, in mature MoDCs, targeting MR also led to efficient cross presentation (Figure 4C). Targeting MR in mature BDCA1+ DCs resulted in little to no cross presentation, probably due to the absence of MR on the surface on maturation (Figure 1A). In both immature and mature DCs, targeting DEC205 resulted in similarly low levels of cross presentation (supplemental Figure 3) and was unable to induce cross presentation as efficiently as targeting MR or CD40, except at the highest antibody concentrations used in immature MoDCs (Figure 4B). Figure 4D shows donor-matched preprocessed HLA-A*0201+ peptide controls, with mature DCs presenting surface-loaded influenza M1 peptide more efficiently than immature DCs, in line with their higher surface expression of MHC class I. CD40 antibodies coupled to the CMV pp65 peptide also resulted in superior cross presentation compared with DEC205-pp65 antibodies (Figure 4E). Thus, receptor-mediated delivery to early endosomes is a relevant determinant of cross presentation efficiency for multiple epitopes. We determined whether a cytosolic intermediate was involved for antibody-targeted cross presentation to both early and late endosomes by inhibiting proteasomal activity. Cross presentation via all 3 receptors required proteasomal degradation for the generation of peptides, whereas surface peptide loading and presentation remained intact in the presence of epoxomicin (Figure 4F).
Having shown that MHC class I cross presentation was superior when targeting CD40 or MR over DEC205, we next asked if the same held true for processing and presentation on MHC class II. We chemically coupled anti-CD40 and anti-DEC205 to an extended MHC class II peptide from the NY-ESO protein that had a cysteine-to-alanine mutation to facilitate conjugation (Figure 5A mutated residue bolded). The mutation did not appear to reduce the ability of the peptide to be recognized by NY-ESO–specific T cells (Figure 5B). Therefore, we next incubated BDCA1+ DCs with the antibody conjugates and cocultured DCs together with a NY-ESO peptide–specific CD4 T-cell clone; activation was measured by monitoring the production of 3 cytokines (IFNγ, IL-2, and TNFα). As found for cross presentation of antigens on MHC class I, anti-CD40 was far more efficient at mediating the presentation of a NY-ESO MHC class II epitope compared to anti-DEC205 over a 100-fold range of antibody concentrations (Figure 5C-E) and at different ratios of DCs to T cells (Figure 5F). Thus, antibody targeting to CD40 is not only superior for cross presentation but also for MHC class II presentation.
Targeting CD40 results in superior MHC class II presentation than targeting DEC205. (A) NY-ESO–extended peptide sequence. The amino acid change from cysteine → alanine is indicated in bold. (B) DPB1*0401+ BDCA1+ DCs were incubated with indicated doses of irrelevant HIV reverse transcriptase peptide (HIV RTp), WT NY-ESO peptide (WT NYESOp), or C → A mutated NY-ESO peptide (mut NYESOp) for 1.5 hours, followed by washing and coculture with an NY-ESO peptide–specific CD4+ T-cell clone. Percentage of CD4+ T cells with intracellular IFNγ results are shown as 1 representative of 3 independent DC donors. (C-E) Antibody-NY-ESO peptide conjugates (0.1-10 μg/mL) were fed to BDCA1− DCs for 1.5 hours, followed by washing and coculture with an NY-ESO–specific CD4+ T-cell clone at a ratio of DCs to T cells of 1:1, and subsequent staining for intracellular cytokines. The percentage of CD4+ T cells that are positive for (C) IFNγ, (D) IL-2, and (E) TNFα are depicted. One representative of 3 independent DC donors is shown. (F) As in panels C through E, 1 μg/mL antibody–NY-ESO conjugates were used with varying ratios of DCs to T cells. The percentage of CD4+ T cells that are positive for IFNγ is depicted. One representative of 3 independent DC donors is shown.
Targeting CD40 results in superior MHC class II presentation than targeting DEC205. (A) NY-ESO–extended peptide sequence. The amino acid change from cysteine → alanine is indicated in bold. (B) DPB1*0401+ BDCA1+ DCs were incubated with indicated doses of irrelevant HIV reverse transcriptase peptide (HIV RTp), WT NY-ESO peptide (WT NYESOp), or C → A mutated NY-ESO peptide (mut NYESOp) for 1.5 hours, followed by washing and coculture with an NY-ESO peptide–specific CD4+ T-cell clone. Percentage of CD4+ T cells with intracellular IFNγ results are shown as 1 representative of 3 independent DC donors. (C-E) Antibody-NY-ESO peptide conjugates (0.1-10 μg/mL) were fed to BDCA1− DCs for 1.5 hours, followed by washing and coculture with an NY-ESO–specific CD4+ T-cell clone at a ratio of DCs to T cells of 1:1, and subsequent staining for intracellular cytokines. The percentage of CD4+ T cells that are positive for (C) IFNγ, (D) IL-2, and (E) TNFα are depicted. One representative of 3 independent DC donors is shown. (F) As in panels C through E, 1 μg/mL antibody–NY-ESO conjugates were used with varying ratios of DCs to T cells. The percentage of CD4+ T cells that are positive for IFNγ is depicted. One representative of 3 independent DC donors is shown.
Taken together, these data indicate that antigen accumulation is not necessarily a good predictor of cross presentation efficiency. Instead, receptors that target to early compartments are quantitatively more adept at delivering antigen into the cross presentation pathway than late endosomes, a finding that also seems to hold true for MHC class II presentation. Interestingly, both endosomal destinations required proteasomal degradation. In addition, a receptor (CD40) that internalizes slowly but targets to early endosomes appeared superior for antigen cross presentation.
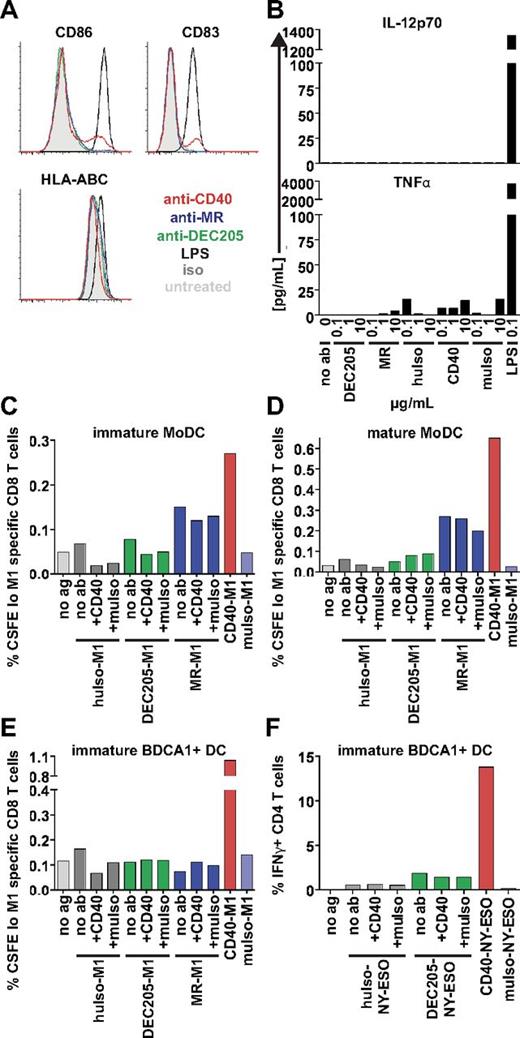
Anti-CD40 does not enhance cross presentation by triggering DC maturation
We next asked if the superiority of anti-CD40 antibody for cross presentation might reflect its ability to generate an activation signal in DCs, especially because CD40 signaling is known to enhance cross presentation in general.6,30 MoDCs were incubated overnight with unlabeled CD40, MR, or DEC205 antibodies and assayed for maturation. Anti-CD40 slightly activated MoDCs, as gauged by CD86 and CD83 up-regulation on a small cell population. HLA-ABC (MHC class I) surface levels remained unaffected by the addition of anti-CD40, indicating that the up-regulation of HLA-ABC by anti-CD40 signaling was not responsible for enhanced cross presentation (Figure 6A). Anti-DEC205 and anti-MR antibodies did not cause up-regulation of any surface markers (Figure 6A). Analysis of supernatants collected from MoDCs treated overnight with these antibodies showed nondetectable levels of IL-12p70 and minimal amounts of TNFα in response to anti-CD40 (Figure 6B). These experiments indicate that the addition of the anti-CD40 antibody used here (S2C6) induced a low but detectable level of DC activation, consistent with this antibody's previous characterization as a weak agonist in vitro.
Anti-CD40 does not enhance cross presentation of MR or DEC205. (A) We added 1 μg/mL anti-CD40 (S2C6), anti-MR (B11), anti-DEC205 (3G9), isotype (iso), or 200 ng/mL LPS to MoDCs overnight. After overnight culture, supernatants were harvested, and DCs were labeled for surface markers that are normally up-regulated after maturation, as indicated. One representative of 3 independent donors is shown. (B) Supernatants from panel A were analyzed for cytokine production with the use of Luminex technology. IL-12p70 levels were insignificant, except for LPS controls. (C-D) MoDCs or (E) BDCA1+ DCs were incubated with 1 μg/mL antibody-M1 peptide conjugates alone or with antibody-M1 conjugates plus 1 μg/mL unconjugated CD40 (S2C6) antibody or 1 μg/mL isotype (muIso) together for 4-6 hours, followed by washing and coculture with autologous CFSE-labeled CD8+ T cells. Graphs depict frequencies of total CFSElo and Influenza M1 (58-66) specific CD8 T cells. One representative of 3 independent donors is shown. (F) BDCA1+ DCs were incubated with 1 μg/mL DEC205–NY-ESO peptide conjugates alone or with DEC205–NY-ESO peptide conjugates plus 1 μg/mL unconjugated CD40 (S2C6) antibody or 1 μg/mL isotype (muIso) together for 1.5 hours, followed by washing and coculture with an NY-ESO–specific CD4+ T-cell clone. Graphs depict percentage of IFNγ+ CD4+ T cells. One representative of 2 independent DC donors is shown.
Anti-CD40 does not enhance cross presentation of MR or DEC205. (A) We added 1 μg/mL anti-CD40 (S2C6), anti-MR (B11), anti-DEC205 (3G9), isotype (iso), or 200 ng/mL LPS to MoDCs overnight. After overnight culture, supernatants were harvested, and DCs were labeled for surface markers that are normally up-regulated after maturation, as indicated. One representative of 3 independent donors is shown. (B) Supernatants from panel A were analyzed for cytokine production with the use of Luminex technology. IL-12p70 levels were insignificant, except for LPS controls. (C-D) MoDCs or (E) BDCA1+ DCs were incubated with 1 μg/mL antibody-M1 peptide conjugates alone or with antibody-M1 conjugates plus 1 μg/mL unconjugated CD40 (S2C6) antibody or 1 μg/mL isotype (muIso) together for 4-6 hours, followed by washing and coculture with autologous CFSE-labeled CD8+ T cells. Graphs depict frequencies of total CFSElo and Influenza M1 (58-66) specific CD8 T cells. One representative of 3 independent donors is shown. (F) BDCA1+ DCs were incubated with 1 μg/mL DEC205–NY-ESO peptide conjugates alone or with DEC205–NY-ESO peptide conjugates plus 1 μg/mL unconjugated CD40 (S2C6) antibody or 1 μg/mL isotype (muIso) together for 1.5 hours, followed by washing and coculture with an NY-ESO–specific CD4+ T-cell clone. Graphs depict percentage of IFNγ+ CD4+ T cells. One representative of 2 independent DC donors is shown.
To determine whether the low level of CD40-mediated DC activation might itself enhance cross presentation, we asked if the addition of anti-CD40 antibodies would enhance cross presentation by the anti–DEC205-M1 and anti–MR-M1 antibody conjugates. DCs were incubated with either anti–DEC205-M1 or anti–MR-M1 together with 1 μg/mL unconjugated anti-CD40 or isotype control during the antigen uptake phase. In immature and mature MoDCs (Figure 6C-D) and in immature BDCA1+ DCs (Figure 6E), the addition of CD40 antibodies did not enhance DEC205 cross presentation. In co-uptake experiments, we observed that both MR and CD40 probably trafficked through the same compartments (data not shown), and it is thought that antigen presentation is enhanced when both antigen and agonist originate from the same compartment.31 However, when we coadministered MR-M1 and anti-CD40, this did not enhance cross presentation via MR (Figure 6C-E), indicating that the addition of this anti-CD40 antibody did not enhance cross presentation by triggering DC activation. Indeed, adding anti-CD40 antibodies also did not improve MHC class II presentation by DEC205 (Figure 6F), indicating that any anti-CD40 signal imparted was negligible for both antigen presentation pathways tested.
DEC205 antibodies are degraded over time, whereas CD40 and MR antibodies remain stable
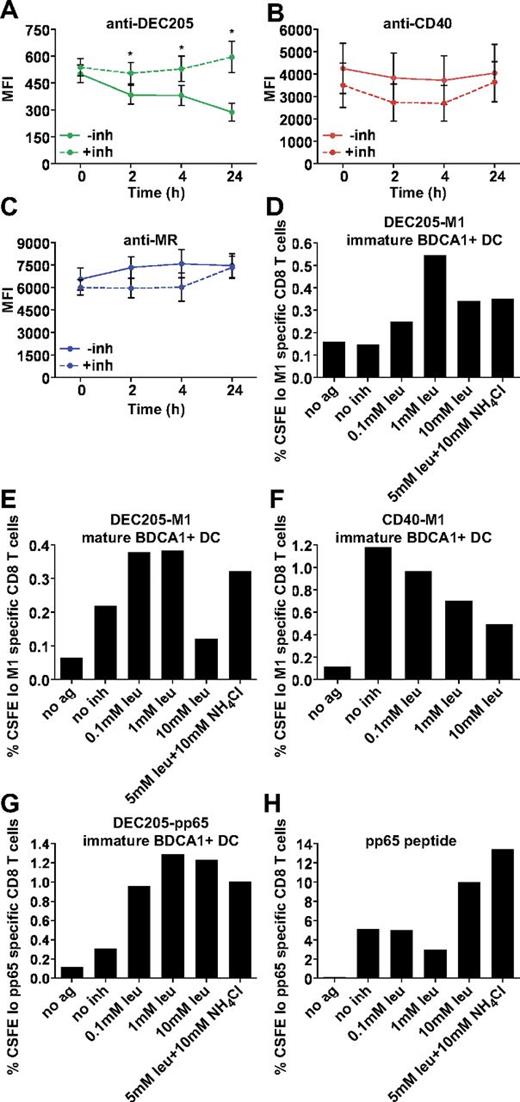
Having ruled out a role for CD40 signaling in enhancing cross presentation and having established that the extent of antigen uptake is not a key factor in cross presentation efficiency, we wanted to understand whether differences in antibody degradation were responsible, as suggested by our earlier accumulation experiments. To more directly monitor antibody degradation, we pulsed MoDCs with Alexa 488–conjugated antibodies for 1 hour at 4°C or 37°C, followed by washing and chasing for the indicated times. After 24 hours, there was a significant loss of anti-DEC205 signal (Figure 7A), whereas both anti-CD40 and anti-MR signal remained relatively constant (Figure 7B-C), indicating that anti-DEC205 was exposed to greater degradative forces. Parallel experiments were performed in cells treated with leupeptin and NH4Cl (inh) to inhibit endosomal-lysosomal proteolysis. Although we did not see an appreciable change in anti-MR and anti-CD40 fluorescence levels on inhibitor addition (Figure 1A-C), the inhibitors did rescue the anti-DEC205 antibody signal, consistent with this antibody having been delivered to late endosomes and subjected to degradation.
DEC205-targeted cross presentation is rescued by the addition of protease and acidification inhibitors. Alexa 488 (4 μg/mL) covalently conjugated (A) anti-DEC205 (3G9), (B) anti-CD40 (S2C6), or (C) anti-MR (B11) antibodies were internalized by MoDCs for 1 hour at 37°C. Cells were washed extensively and chased for indicated times with or without leupeptin and NH4Cl (inh), followed by fixation and analysis by flow cytometry. Graphs depict the mean MFI ± SEM of 3 independent donors. MFI differences in the absence or presence of inhibitors were assessed with the paired t test, and statistically significant differences were depicted, *P < .05. (D) Immature or (E) mature BDCA1+ DCs were incubated with 1 μg/mL DEC205-M1 conjugates with or without indicated inhibitors for 4-6 hours, followed by washing and coculture with autologous CFSE-labeled CD8+ T cells. Graphs depict frequencies of CFSElo, influenza M1 (58-66) specific CD8+ T cells. One representative of 3 independent donors is shown (D) and 1 representative of 2 independent donors is shown (E). (F) Immature BDCA1+ DCs were incubated with 1 μg/mL CD40-M1 conjugates with or without indicated inhibitors for 4-6 hours, followed by washing and coculture with autologous CFSE-labeled CD8+ T cells. Graphs depict frequencies of total CFSElo and Influenza M1 (58-66) specific CD8+ T cells. One representative of 3 independent donors is shown. (G) BDCA1+ DCs were incubated with 1 μg/mL DEC205-pp65 conjugates with or without indicated inhibitors for 4-6 hours, followed by washing and coculture with autologous CFSE-labeled CD8+ T cells. Graphs depict frequencies of CFSElo, CMV pp65 (495-503) specific CD8+ T cells. One representative of 2 independent donors is shown. (H) CMV pp65 (495-503) nonextended peptide control (25 ng/mL).
DEC205-targeted cross presentation is rescued by the addition of protease and acidification inhibitors. Alexa 488 (4 μg/mL) covalently conjugated (A) anti-DEC205 (3G9), (B) anti-CD40 (S2C6), or (C) anti-MR (B11) antibodies were internalized by MoDCs for 1 hour at 37°C. Cells were washed extensively and chased for indicated times with or without leupeptin and NH4Cl (inh), followed by fixation and analysis by flow cytometry. Graphs depict the mean MFI ± SEM of 3 independent donors. MFI differences in the absence or presence of inhibitors were assessed with the paired t test, and statistically significant differences were depicted, *P < .05. (D) Immature or (E) mature BDCA1+ DCs were incubated with 1 μg/mL DEC205-M1 conjugates with or without indicated inhibitors for 4-6 hours, followed by washing and coculture with autologous CFSE-labeled CD8+ T cells. Graphs depict frequencies of CFSElo, influenza M1 (58-66) specific CD8+ T cells. One representative of 3 independent donors is shown (D) and 1 representative of 2 independent donors is shown (E). (F) Immature BDCA1+ DCs were incubated with 1 μg/mL CD40-M1 conjugates with or without indicated inhibitors for 4-6 hours, followed by washing and coculture with autologous CFSE-labeled CD8+ T cells. Graphs depict frequencies of total CFSElo and Influenza M1 (58-66) specific CD8+ T cells. One representative of 3 independent donors is shown. (G) BDCA1+ DCs were incubated with 1 μg/mL DEC205-pp65 conjugates with or without indicated inhibitors for 4-6 hours, followed by washing and coculture with autologous CFSE-labeled CD8+ T cells. Graphs depict frequencies of CFSElo, CMV pp65 (495-503) specific CD8+ T cells. One representative of 2 independent donors is shown. (H) CMV pp65 (495-503) nonextended peptide control (25 ng/mL).
Inhibiting degradation rescues DEC205 targeted cross presentation
Conceivably, delivery to degradative compartments reduces cross presentation by causing intralysosomal degradation of the internalized antibody-peptide conjugates before release into the cytosol for proteasomal processing. Indeed, this difference might be accentuated in our system, which relies on the use of relatively small peptide conjugates that could be more easily digested than intact protein antigens. Resistance to degradation is well known to be important for MHC class II presentation,1,32-34 with antigens more resistant to degradation being more efficiently processed and presented. Our MHC class II data may also reflect the differences in degradative capacity between the endosomal compartments, because targeting CD40 elicited superior CD4+ T-cell responses compared with DEC205 (Figure 5). The same may be true in the case of cross presentation, given studies that show enhancement when antigen-presenting cells were treated with chloroquine.19,35 To examine this possibility, we added protease and acidification inhibitors during the antigen capture phase of our CD8+ T-cell assay. As shown in Figure 7, the addition of protease and acidification inhibitors enhanced cross presentation via DEC205 in both immature (Figure 7D) and mature DCs (Figure 7E). This was true for influenza M1 and CMV pp65 peptide conjugates (Figure 7D,G). Interestingly, addition of increasing concentrations of protease inhibitors reduced cross presentation via CD40 (Figure 7F), indicating that some level of proteolysis was required for escape to the cytosol and subsequent cross presentation. Presentation of unconjugated peptide was largely unaffected by the inhibitor treatments, except at the highest concentrations (Figure 7H). Thus, cross presentation of antigens delivered to highly degradative compartments could be enhanced by inhibiting the degradative capacity of these compartments. In sum, by targeting 3 receptors with distinct endosomal localization patterns, we show that targeting early endosomal receptors with antibody-peptide conjugates results in more efficient cross presentation, due to the lowered degradative capacity of this compartment. In addition, we find that under conditions in which proteases are inhibited, both late and early endosomal compartments are able to support cross presentation, arguing against the existence of specializations unique to a single endosome population.
Discussion
DCs harbor a variety of specializations that together enable their capacity for antigen presentation and the control of T-cell stimulation.1 Although a number of these specializations affect the endosomal and phagosomal apparatus,36,37 the formation of MHC class II–peptide complexes does not appear to be linked to any one intracellular site. The situation is less clear in the case of cross presentation on MHC class I molecules. Although antigen can be cross presented regardless of internalization mode,38-40 whether it must access a specific endosomal compartment was uncertain. Previous reports have implicated early endosomes as essential for cross presentation, based on a qualitative comparison of receptor-mediated versus fluid phase ovalbumin uptake and the possible presence of the TAP2 translocator by immunofluorescence microscopy,16,18 whereas other studies with little emphasis on intracellular trafficking have determined that many receptors are able to cross present equivalently with great efficiency.10,12,21,22
We avoided making any assumptions about which endosomal compartment was essential or what specializations it might possess for cross presentation by using high-affinity antibodies that targeted antigen to distinct compartments. This approach allowed a direct, quantitative assessment of the relative importance of antigen internalization, localization, and degradation in determining the efficiency of cross presentation in 2 different human DC populations. Our results suggest that both early and late endosomal compartments are capable of serving as antigen portals for cytosolic entry and cross presentation. Late compartments appear to be less efficient for some antigens, however, due to a higher concentration of lysosomal enzymes, which degrade antigens before they can be released into the cytosol. Accordingly, we find that the rapidity of degradation can explain at least some of the compartment-specific differences in cross presentation. The fact that inhibiting proteolysis enhances the ability of late endosomes and lysosomes to allow cross presentation of accumulated antigens also means that it is unlikely that early endosomes alone contain the specializations required for antigen egress or for re-importation for loading onto endosomal MHC class I molecules, as proposed previously.18
Our ability to detect a relationship between degradation and presentation from late compartments may reflect the nature of the antigen delivery platform we have developed, that is, the use of extended peptides chemically coupled to antireceptor antibodies. Previous work has relied mostly on the use of heavy chain fusions of full-length antigens,9,10 which may be inherently more resistant to degradation. In our hands, such fusions also have a significant tendency to aggregate and to be proteolysed during production, which would complicate potential scale-up for application in human patients. Our peptide platform offers the advantage of scalability and combinatorial flexibility (allowing for delivery of different peptides), although the potential decrease in peptide antigen stability may require the targeting of such constructs to early endosomes. Indeed, protein stability may be a reason why targeting DEC205 is inferior for cross presentation; peptide antigens are probably more susceptible to degradation, which would decrease their ability to survive long enough to escape into the cytosol. The use of more susceptible peptide antigens may also explain why anti-CD40 was superior to anti-DEC205 for targeting MHC class II presentation. Given that an inverse relation between antigen stability and formation of peptide–MHC class II complexes is known to exist,33 it is perhaps not too surprising that a similar situation might exist for cross-presented antigens.
We were, however, surprised by the observation that targeting CD40 mediated more efficient cross presentation than MR. Both receptors targeted internalized peptide-antibody conjugates to early endosomes, but CD40 was substantially less efficient at antibody uptake. The enhanced cross presentation was not because of maturation effects of anti-CD40, which has been shown to activate cross presentation by mouse DCs.6,30 The one obvious difference was in the localization of antibodies even after extended time points. Although the bulk of anti-MR was internalized, most anti-CD40 remained at the plasma membrane from which it was presumably continuously but more slowly internalized, or to which it was more rapidly recycled. Conceivably, maximum antigen stability may be achieved when antibodies are internalized slowly to early and recycling endosomes. This limits the time that the antibody-peptide conjugate spends intracellularly, where early endosomal proteases (albeit fewer) gain access to antigens41,42 and provides a continuous “time-release” pool of antigen that might be used over extended periods for the continuous formation of peptide–MHC class I complexes. Indeed, preliminary cross presentation data from another slowly internalizing receptor, CD11c, appears to support this hypothesis (B.C. and I.M., unpublished data, February 1, 2012). Whatever the underlying mechanism, this observation was unexpected and indicates that rapid endocytosis and antigen accumulation may not always be the best criteria for choosing receptors to target vaccines to DCs.
Our data may additionally point to some cell type–specific differences in cross presentation efficiency. Both DC types examined exhibited similar patterns of receptor expression, localization, antibody accumulation, and cross presentation. However, although MoDCs were more endocytic overall, they also appeared to be more degradative, especially when we assayed for DEC205 targeting. We generally see that immature MoDCs exhibit lower cross presentation efficiency than BDCA1+ DCs, and one reason for this may be because of higher protease levels in MoDCs compared with BDCA1+ DCs.28,29 The existence of distinct DC subsets that have variable capacities for cross presentation may mean that any such specializations could also be cell type specific. Indeed, recent in vivo work in the mouse has shown that as long as a given antibody (DEC205, Langerin, CLEC9A/DNGR-1) can target CD8α+ cross-presenting DCs, cross presentation will occur.10 Recent studies on DC subtypes have implicated BDCA3+ DCs as the human equivalent of the mouse CD8α+ DCs,43-46 a DC type that is key for efficient cross presentation in vivo in mice.47-49 Initial data from these groups indicate that BDCA3+ DCs are superior for cross presentation, compared with BDCA1+ DCs. Our data indicate that degradation of antigen affects the efficiency of cross presentation by human DCs, including BDCA1+ DCs, and that slower antigen internalization to early endosomes may be superior. Therefore, it may be possible that differential antigen handling by BDCA3+ DCs may be the reason for these differences, potentially through altered antigen accumulation or degradation or perhaps through increased access to the cytosol.
Further studies to elucidate the requirements for efficient cross presentation will be key for efficient DC targeting. Because we see that multiple DC types are able to cross present, it will be advantageous to target greater than 1 type of DC simultaneously to elicit the most efficient CD8+ T-cell responses. On the basis of our results, this will best be accomplished not only by taking into account surface receptor expression but also by determining the intracellular localization and degradation of the trafficking antibodies of interest.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Genentech Research Blood Program and Blood Centers of the Pacific for donor coordination and leukapheresis, as well as Laurie Gilmour and Yelena Dayter for technical assistance with elutriations. They especially thank members of the Mellman laboratory for advice and fruitful discussions, and Peter Ebert for the critical reading of this manuscript.
This article is dedicated to the memory of our mentor and dear friend, Ralph Steinman.
Authorship
Contribution: B.C. and I.M. designed the experiments with help from A.S.-S.; B.C. performed the experiments; B.C. and I.M. analyzed the data; A.S.S., L.D., and I.M. supervised the project; L.C. assisted with primary DC isolation; C.C. assisted with imaging analysis; R.V. and B.-C.L. generated the antibody-peptide conjugates; J.W. and T.K. provided the B11 and 3G9 antibodies; B.C. and I.M. wrote the paper; and all authors approved this manuscript.
Conflict-of-interest disclosure: B.C., L.C., C.C., R.V., B.-C.L., L.D., and I.M. are employees of Genentech. J.W. and T.K. are employees of Celldex Therapeutics. The remaining author declares no competing financial interests.
Correspondence: Ira Mellman, Genentech, 1 DNA Way, Mail Stop 212, South San Francisco, CA 94080; e-mail: mellman.ira@gene.com.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal