One-quarter of acute promyelocytic leukemia (APL) patients develop resistance to all-trans retinoic acid (ATRA)/chemotherapy (CT). In this issue of Blood, Gallagher et al report the associations of PML-RARα mutations, FLT3 mutations, and additional chromosome abnormalities (ACAs) in relapsed APL.1
Intrinsic or acquired resistance to anticancer drugs can arise from a variety of factors including the development of mutations in drug targets and additional genetic abnormalities. In APL, which is driven by the t(15;17)–generating PML-RARα, ATRA in combination with CT achieves a complete remission rate of 90% and a 5-year disease-free survival of 74%.2,3 However, ATRA resistance has been reported for 2 decades and was shown to be associated with increased catabolism and decreased delivery to cell nucleus of ATRA, as well as mutations in the ligand-binding domain (LBD) of the RARα portion of the fusion protein.4,5 In this study, Gallagher et al further dissect the potential association of PML-RARα LBD mutations (PRα/LBD+), FLT3 mutations, and ACAs in relapsed APL on ATRA/CT (see figure).1
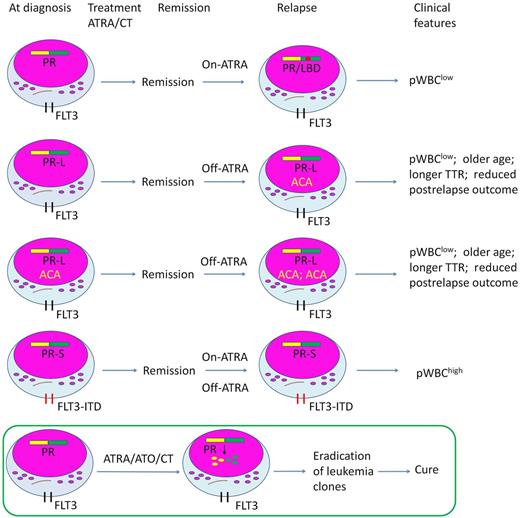
Association of PML-RARα LBD mutations (PRα/LBD+), FLT3 mutations and additional chromosome abnormalities (ACAs) in relapsed acute promyelocytic leukemia (APL) on all-trans retinoic acid (ATRA)/chemotherapy (CT). TTR indicates time to relapse. The bottom panel represents the more recently developed ATRA/arsenic trioxide (ATO) combination therapy for the newly diagnosed APL, which can eradicate the leukemia clone through induction of PML-RARα degradation and lead to a long-term clinical remission in the overwhelming majority of patients.
Association of PML-RARα LBD mutations (PRα/LBD+), FLT3 mutations and additional chromosome abnormalities (ACAs) in relapsed acute promyelocytic leukemia (APL) on all-trans retinoic acid (ATRA)/chemotherapy (CT). TTR indicates time to relapse. The bottom panel represents the more recently developed ATRA/arsenic trioxide (ATO) combination therapy for the newly diagnosed APL, which can eradicate the leukemia clone through induction of PML-RARα degradation and lead to a long-term clinical remission in the overwhelming majority of patients.
The authors show that among 45 relapsed patients from the ATRA/CT treatment group, 18 cases harbor PRα/LBD+ (40%), 7 of whom (39%) relapsed more than 30 days after last ATRA dose (off ATRA) selection pressure, suggesting a possible active role of PRα/LBD+. Indeed, Gallagher et al observed in 2 cases the selection of a pre-existing mutant subclone by ATRA that lead to relapse on or off ATRA treatment.6 Unlike PRα/LBD+, the incidence and quantitation of FLT3-ITD+ are not increased during relapse without any evidence of influence of ATRA treatment. The fact that all FLT3-ITD+ patients who relapsed off ATRA lacked PRα/LBD+, and most FLT3-ITD+ patients (83%) who relapsed on ATRA had a coincident PRα/LBD+, suggests that ATRA could not eliminate the double-mutant subclone. Exclusive ACAs are identified at diagnosis, and are significantly increased (2-fold) at relapse (29% to 62%). Structural chromosome changes are predominantly newly present at relapse and differ from ACA at diagnosis. Interestingly, despite the heterogeneity of ACAs, they are associated with a phenotype of pWBClow and L-isoform, if relapse occurred off ATRA. FLT3-ITD+ as a mechanism leading to off-ATRA disease progression may be proved by the observation that ACA-PRα/LBD+ is negatively associated with FLT3-ITD+, which presents an opposite phenotype of pWBChigh and S-isoform. However, in on-ATRA relapsed patients, the above associations are not apparent, suggesting a distinct different mechanism of resistance and progression. In prognostic analysis, only the presence of ACA at relapse is associated with reduced postrelapse outcome. In the patients with PRα/LBD+, missense mutations of G289, deletion mutations, and pediatric/juvenile type of mutations affecting Y208 or R217 are associated with aggressive disease. FLT3-ITD+ and FLT3/D835+ at relapse do not affect treatment outcome.
PML-RARα is the key driver of leukemogenesis of APL, which exerts a dominant-negative effect on its parental proteins that involves the process of myeloid differentiation, apoptosis, and DNA replication and repair.3,7 PML-RARα also provides the direct pharmaceutical target of 2 effective agents for APL, ATRA and arsenic trioxide (ATO): arsenic binds to the RING-B Box-Coiled-Coil (RBCC) domain of PML moiety,8 whereas ATRA targets the RARα moiety of PML-RARα9 (see figure). According to different mechanism of 2 agents, potentially PRα/LBD+–induced resistance or relapse could be overcome by ATO; on the contrary, rare but real existing PML mutation10 could be reversed by ATRA. In the clinical setting the combination of ATRA and ATO could lead to a curable status of APL. The relationship of PRα/LBD+, FLT3-ITD, ACA, and even PML mutation and their impact on the prognosis of APL would be more comprehensive with the incorporation of ATO, but remains an open issue. In addition, the mechanisms underlying the development of PRα/LBD+ in abnormal promyelocytes on ATRA and whether or not the PRα/LBD+ could undergo ATRA-induced catabolism warrant further investigation.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■