In this issue of Blood, Ye and colleagues demonstrate that human Foxp3+ regulatory T (Treg) cells suppress target T cells by inducing senescence, and this endows them with suppressive functions.1
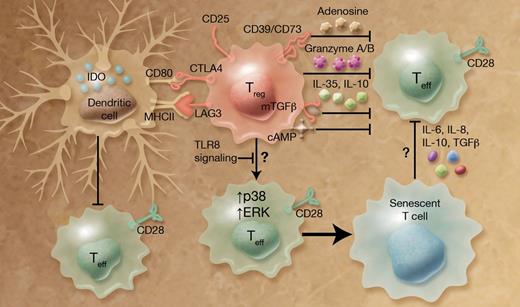
Treg cells are pivotal in maintaining self-tolerance and immune homeostasis, but can also inhibit immune responses against cancer and infection.2 To tap the potential of Treg cells in clinical application, it is crucial to understand the effector mechanisms by which Treg cells exert their suppressive functions. Many molecular mechanisms have been proposed (see figure and reviewed in Shevach3 and Vignali et al4 ). Briefly, suppression can be carried out by: (1) inhibitory cytokines, such as TGFβ (including membrane-tethered mTGFβ), IL-10, and IL-35; (2) cytolytic enzymes, such as granzyme A/B; (3) metabolic disruption including consumption of local IL-2 by CD25, generation of pericellular adenosine by ectoenzyme CD39 and CD73, and generation of cyclic adenosine monophosphate (cAMP); and (4) targeting antigen presenting cells, particularly dendritic cells (DCs). For example, interaction of CTLA4, which is highly expressed on Treg cells, with CD80/CD86 on DCs leads to the induction of indoleamine 2, 3-dioxygenase (IDO), an immunosuppressive molecule.
Treg cells use multiple mechanisms to suppress effector T (Teff) cells, including the newly identified process that involves induction of target cell senescence. Treg cells produce inhibitory cytokines (such as TGFβ, IL-10, and IL-35), cytolytic enzymes (such as granzyme A/B), and suppressive molecules (such as cAMP). They also express surface receptors, including CD25 (IL-2R), CD39, CD73, CTLA4, and LAG3, important for immune regulation. Treg cells induce senescence of responder cells by up-regulation of p38 and ERK1/2 activities, and this in turn endows them with suppressive functions, thereby reinforcing Treg-mediated suppression. Stimulation with TLR8 ligands abrogates the ability of Treg cells to induce senescence. Professional illustration by Alice Y. Chen.
Treg cells use multiple mechanisms to suppress effector T (Teff) cells, including the newly identified process that involves induction of target cell senescence. Treg cells produce inhibitory cytokines (such as TGFβ, IL-10, and IL-35), cytolytic enzymes (such as granzyme A/B), and suppressive molecules (such as cAMP). They also express surface receptors, including CD25 (IL-2R), CD39, CD73, CTLA4, and LAG3, important for immune regulation. Treg cells induce senescence of responder cells by up-regulation of p38 and ERK1/2 activities, and this in turn endows them with suppressive functions, thereby reinforcing Treg-mediated suppression. Stimulation with TLR8 ligands abrogates the ability of Treg cells to induce senescence. Professional illustration by Alice Y. Chen.
Despite these proposed molecular mechanisms, relatively little is known about the cellular events during Treg-mediated suppression. In particular, the fate of responder T cells is not fully understood, although apoptosis has been observed in these cells.5 Another unresolved question is how the responder cells interact with Treg cells and other immune cells. Indeed, there have been publications showing that naive T cells can be turned into suppressor cells by Treg cells and therefore amplify Treg-mediated suppression, a phenomenon called infectious tolerance.6,7 Lastly, many of these proposed mechanisms are derived from studies in the murine systems, and it is not clear whether human Treg cells use the same mechanisms.
Ye et al examined the fate of suppressed T cells using human Treg cells and responder (both naive and memory) T cells. Interestingly, they show that responder T cells do not undergo apoptosis, but instead become senescent, which is characterized by permanent cell cycle arrest and loss of surface CD27 and CD28 expression.1 Loss of CD28 is a hallmark of T cells from elderly people or patients with chronic viral infection and inflammatory diseases. Notably, this effect only occurs in primates, but not in laboratory mice.8 Hence it is possible that this Treg-enforced senescence is a novel mechanism used by human Treg cells, but not murine Treg cells. Another important conclusion from Ye et al is that the Treg-induced senescent cells can in turn suppress naive T-cell proliferation.1 These data therefore provide new insight into infectious tolerance, which could play an important role in human physiology.9
Ye and colleagues further investigated the molecular pathways involved in Treg-induced senescence. They demonstrate that p38 and ERK1/2 MAP kinases, but not JNK, are activated in responder cells. Blocking p38 or ERK1/2 by pharmacologic inhibitors or siRNA knockdown prevents Treg-induced senescence. On the Treg side, continuing the authors' previous findings,10 they now show that stimulation with TLR8 ligands, but not other TLR ligands, abrogates the ability of Treg cells to induce target cell senescence. These results suggest that human Treg-mediated suppression can be manipulated pharmacologically either on Treg cells or on responder cells, which could have important clinical implications.
This elegant study also raises several interesting questions. What is the mechanism through which human Treg cells exert their senescence-inducing function, and is it through soluble factors or cell contact dependent processes? Similarly, although the data suggest that cytokines produced by Treg-induced senescent T cells might be responsible for their suppressive functions, the detailed mechanism remains to be identified. Further, it will be interesting to ascertain whether alterations of Treg-enforced senescence contribute to human autoimmune disorders and other immune-mediated diseases. No matter what is found in future studies, the present work reveals new territory that will undoubtedly provide more insight into the potentially lifesaving field of human Treg cells.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal