William James, the 19th century American philosopher, famously asserted, “To study the abnormal is the best way of understanding the normal.” In this issue of Blood, using a distinctly non-Jamesian approach, Bellissimo and colleagues shed new light on our understanding of normal genetic variation in von Willebrand factor (VWF) through the study of ethnically diverse healthy controls and, in doing so, exonerate a number of benign sequence variants previously regarded as pathogenic mutations.1
Since the VWF gene was first characterized,2 tremendous strides have been made in our understanding of the pathophysiology and molecular genetics of type 1 von Willebrand disease (VWD), a common inherited bleeding disorder caused by quantitative deficiency of the VWF protein. The VWF gene extends over 178 kilobases on chromosome 12 and is composed of 52 exons, encoding a large precursor protein of 2813 amino acids. The precursor protein is synthesized in megakaryocytes and endothelial cells and undergoes a series of posttranslational modifications to produce the 2050 amino acid VWF monomer. Monomers dimerize and further polymerize via disulfide bonds to form large multimers ranging from 0.5 to 10 million Daltons in size. The mature VWF multimers stabilize factor VIII and mediate platelet adhesion at sites of vascular injury, functions carried out by conserved structural domains.3
The VWF gene is highly polymorphic4 with a number of single nucleotide substitutions, insertions, and deletions reported in both coding and noncoding regions. In addition, several hundred candidate mutations have been described in patients with VWD.5 There is a strong impetus to connect these underlying genetic defects to disease pathophysiology and clinical phenotype; doing so holds promise for more precise diagnostics and targeted therapeutics. However, distinguishing between causative mutations and neutral sequence variants remains challenging.6
Until now, investigators have addressed this challenge largely through the study of patients with clinical and laboratory evidence of VWD. Bellissimo and colleagues have taken a different approach. They analyzed the coding DNA sequences and corresponding standard VWF clinical laboratory tests in 184 healthy individuals with a negative bleeding history recruited from 7 centers across the US.1 The study population included 118 whites and 66 blacks. Sequence analysis revealed 21 new variations, highlighting the polymorphic nature of the VWF gene. Remarkably, an additional 14 sequence variants previously implicated as pathogenic mutations, primarily in type 1 VWD, were identified in the study. Individuals carrying these alleles had a negative bleeding history and all but 1 had normal VWF clinical laboratory testing, suggesting that many of these putative disease-causing mutations are likely to be benign sequence variations, although incomplete penetrance remains an alternative explanation. Analysis of healthy black subjects proved particularly revealing. Thirteen hitherto undescribed variants and 10 sequence variations previously classified as pathogenic occurred exclusively in this cohort, likely reflecting the greater genetic diversity observed in individuals of African origin7 and underrepresentation of this group in previous studies of VWF genetics.
The current study represents the first analysis of VWF genetic variation in a large number of ethnically diverse healthy controls and exposes a potential pitfall of genetic testing in type 1 VWD and other genetically heterogeneous disorders, namely the uncertainty inherent in classifying a given variant as pathogenic. From the ongoing 1000 Genomes Project and the International HapMap Consortium, in which genomic variation in major population groups from Europe, East Asia, South Asia, West Africa, and the Americas are being studied, it is estimated that an individual genome varies from the reference genome sequence at 10 000 to 11 000 nonsynonymous and 10 000 to 12 000 synonymous sites.7–8 Importantly, amid this staggering number of polymorphisms across the genome, only an estimated 340 to 400 sequence variants per individual are predicted to cause significant gene disruption by altering splice sites, creating premature stop codons, or leading to shift of reading frame, and a smaller fraction still are thought to be of clinical relevance.7 The challenge of teasing out pathogenic mutations in VWF from this haystack of polymorphisms is further complicated by the many nongenetic and extra-allelic genetic factors that influence VWF levels including ABO blood group, race, age, stress, body-mass index, pregnancy, and medications.9–10
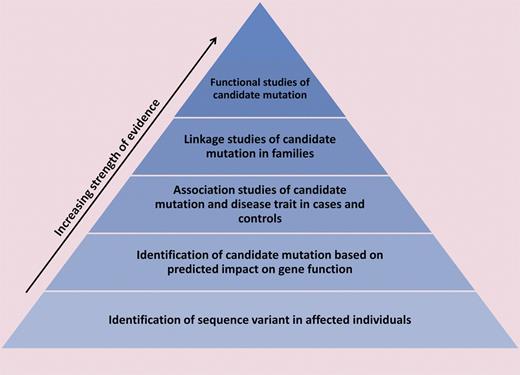
To provide a framework for evaluating the likelihood of pathogenicity of a given variant, we propose a hierarchical model of evidence for genotype-phenotype correlation, wherein population-based studies of genetic variants and identification of candidate mutations in affected individuals provide relatively weak evidence and functional studies of candidate mutations provide strong evidence for the existence of a genotype-phenotype relationship (see figure). The pathogenicity of previously reported mutations in VWF should be re-evaluated in the context of such a framework. Moreover, it may be beneficial to include the strength of evidence alongside each putative mutation in mutation repositories such as the VWF online database.5
A hierarchical model of evidence for establishing the pathogenicity of a sequence variant is shown. Identification of a novel variant in an affected individual provides relatively weak evidence because it does not address the possibility that the variant is benign. Somewhat stronger evidence comes from prediction of a variant's impact on gene function, for example, changes in protein-coding sequence or known regulatory regions or disruption of an evolutionarily conserved domain. Association studies of phenotype and candidate mutations in cases and controls and linkage studies of candidate mutations within families offer further support for a variant's clinical significance. The most conclusive evidence of pathogenicity is provided by functional studies of sequence variants: for example, introduction of a candidate mutation in vivo recapitulates the disease phenotype or affects protein function in an in vitro assay. As demonstrated by Bellissimo et al, undergirding all of these studies are investigations of ethnically diverse normal populations, which help to illuminate normal genetic variation.5
A hierarchical model of evidence for establishing the pathogenicity of a sequence variant is shown. Identification of a novel variant in an affected individual provides relatively weak evidence because it does not address the possibility that the variant is benign. Somewhat stronger evidence comes from prediction of a variant's impact on gene function, for example, changes in protein-coding sequence or known regulatory regions or disruption of an evolutionarily conserved domain. Association studies of phenotype and candidate mutations in cases and controls and linkage studies of candidate mutations within families offer further support for a variant's clinical significance. The most conclusive evidence of pathogenicity is provided by functional studies of sequence variants: for example, introduction of a candidate mutation in vivo recapitulates the disease phenotype or affects protein function in an in vitro assay. As demonstrated by Bellissimo et al, undergirding all of these studies are investigations of ethnically diverse normal populations, which help to illuminate normal genetic variation.5
The provocative results of Bellissimo et al add valuable information to our understanding of the molecular genetics of VWF and VWD. More fundamentally, they underscore the importance of studying ethnically diverse healthy controls, both to expand our knowledge of normal genetic variation and to distinguish pathogenic changes from benign variants. If William James were alive in the genomic age, he might wish to addend his famous aphorism: “To study the normal is a keystone for understanding the abnormal.”
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■