Abstract
Steady-state hematopoiesis is altered on infection, but the cellular and molecular mechanisms driving these changes are largely unknown. Modulation of hematopoiesis is essential to increase the output of the appropriate type of effector cell required to combat the invading pathogen. In the present study, we demonstrate that the pro-inflammatory cytokine IFNγ is involved in orchestrating inflammation-induced myelopoiesis. Using both mouse models and in vitro assays, we show that IFNγ induces the differentiation of monocytes over neutrophils at the level of myeloid progenitors. Infection with lymphocytic choriomeningitis virus induces monopoiesis in wild-type mice, but causes increased neutrophil production in IFNγ−/− mice. We demonstrate that IFNγ enhances the expression of the monopoiesis-inducing transcription factors IRF8 and PU.1 in myeloid progenitor cells, whereas it reduces G-CSF–driven neutrophil differentiation via a SOCS3-dependent inhibition of STAT3 phosphorylation. These results establish a critical role for IFNγ in directing monocyte versus neutrophil development during immune activation.
Introduction
Tightly regulated proliferation and differentiation of hematopoietic progenitors in the BM is critical for maintaining homeostatic levels of peripheral blood cells and is controlled by intrinsic and extrinsic factors. Immunologic stress conditions such as infection induce changes in the magnitude and composition of hematopoietic output that are essential in meeting the increased demand for immune cells and in inducing the proper immune defense against invading pathogens.1 It has been demonstrated that infection with the extracellular fungal pathogen Candida albicans induces peripheral neutrophilia and an increase in BM neutrophils.2 Conversely, increased production of monocytes has shown to be essential in controlling Listeria monocytogenes and Toxoplasma gondii infections.3,4 However, the molecular and cellular mechanisms underlying these hematopoietic changes in myeloid differentiation remain to be elucidated.
Both neutrophils and monocytes are derived from the granulocyte-macrophage progenitor (GMP) and differentiation to either of these myeloid cell types is driven by a particular cytokine.5,6 In steady-state conditions, G-CSF is crucial for maintaining appropriate neutrophil numbers, because loss of G-CSF or its receptor (G-CSFR) decreases the number of circulating neutrophils, whereas injection of G-CSF increases neutrophil numbers.7,8 G-CSFR signaling induces phosphorylation of signal transducer and activator of transcription 3 (STAT3), a member of the STAT family of signaling proteins that regulate gene expression in response to cytokines.9 The occurrence of emergency granulopoiesis has been linked to increased levels of granulopoiesis-supporting cytokines such as G-CSF and is dependent on STAT3 phosphorylation.10,11 Conversely, the production of monocytes is supported by M-CSF and the transcription factors PU.1 and IRF8, which are essential for monocyte differentiation (for review, see Friedman12 ). The development of monocytosis after Listeria infection could be driven by the corresponding increase in M-CSF levels in serum,10 possibly in conjunction with TLR-mediated signals.13 Conversely, the increase of monocytes in a burn sepsis model was associated with the up-regulation of the M-CSF receptor on myeloid progenitor cells,14,15 whereas the occurrence of neutropenia in this model was due to the recognition of lipopolysaccharide by early precursors.16
Evidence is emerging that activated T cells also play an important role in modulating hematopoiesis during an immune response. Large numbers of effector T cells are known to enter the BM parenchyma during viral infections,17 and T cells are thought to modulate hematopoietic progenitors either through direct cell-cell contact18,19 or by the secretion of pro-inflammatory cytokines such as TNFα or IFNγ.20,21 IFNγ is typically produced in response to infection with intracellular pathogens and has important stimulatory functions in innate and adaptive immunity.22 Using a chronic inflammatory mouse model in which transgenic expression of CD70 on B cells (CD70TG mice) induces CD27-driven formation of IFNγ-producing effector T cells, we have previously demonstrated that T cell–derived IFNγ can also suppress the development of B cells,23 erythrocytes,24 and eosinophilic granulocytes.20 Other reports have also shown lineage-specific effects of IFNγ on infection-induced changes in myelopoiesis.25-27
The cellular and molecular mechanism by which IFNγ differentially affects the formation of various types of myeloid cells is largely unknown. In the present study, we investigated if and how IFNγ affects the mechanisms regulating neutrophil and monocyte differentiation at the level of myeloid progenitors. We report that IFNγ promotes monopoiesis and suppresses neutrophil production in the BM. Correspondingly, IFNγ elevates expression of the monocyte-inducing transcription factors PU.1 and IRF8 in GMPs and reduces G-CSF–mediated phosphorylation of STAT3 in a suppressor of cytokine signaling 3 (SOCS3)–dependent manner. These data demonstrate that IFNγ can directly regulate the balance between monocyte and neutrophil production by affecting cytokine responses and the expression of lineage-specific transcription factors in GMPs.
Methods
Mice
Wild-type (WT), IFNγ−/−, CD70TGxIFNγ−/−, and CD70TG C57BL/6 mice were housed under specific-pathogen-free conditions. Animal experiments were approved by the Animal Ethics Committee of the Academic Medical Center in Amsterdam and were performed in accordance with institutional and national guidelines.
Flow cytometry and cell sorting
Single-cell suspensions of BM were obtained by crushing both tibias and femurs using a mortar and pestle and filtering through 40-μm cell strainers. Erythrocytes in the peripheral blood were lysed with an ammonium chloride solution. Purification of common myeloid progenitors (CMPs) and GMPs was performed as described previously.20 Briefly, BM cells were stained with biotin-conjugated Abs against the lineage markers CD4 (GK1.5), CD8α (53-6.7), B220 (RA3-6B2), CD11b (M1/70), Gr1 (RB6-8C5), Ter119 (Ly-76), and lineage cells were depleted using streptavidin microbeads (Miltenyi Biotec) and MACS LS columns (Miltenyi Biotec). Lineage-depleted cells were incubated with Abs for CD34, CD16/32, Sca-1, and c-Kit and progenitors were sorted on a FACSAria (BD Biosciences). Sca-1 could not be included in the identification of myeloid progenitors in CD70TG mice and lymphocytic choriomeningitis virus (LCMV)–infected mice because of a systemic IFNγ-mediated up-regulation of Sca-1.19 Within experiments, similar definitions were used for the progenitors of all mice included.
The Abs used for identification of cells by flow cytometry were: CD62L-PE–Cy7 (MEL-14), IFNγ-APC (XMG1.2), CD34-FITC (RAM34), Gr1-FITC (RB6-8C5), Ly6G-PE (1A8; both from BD Pharmingen), CD115-biotin (AFS98), CD16/32-PE–Cy7 (93), CD11b-APC, CD11b-APC eFluor-780 (M1/70), c-Kit–PE (BD Biosciences), c-Kit-APC eFluor-780 (2B8), Sca-1–PE, and Sca-1-PE–Cy7 (D7), BrdU-FITC (PRB-1), CD127-FITC (A7R34), PD-1–PE (J43), CD8-PE, CD8-PerCP–Cy5.5 (53-6-7), LCMV GP33-41 tetramer-APC, CD4-Alexa Fluor 700 (L3T4), CD4-PE–Cy7 (GK1.5), CD3-APC eFluor-780, BrdU-FITC (PRB-1), and STAT3-Alexa Fluor 647 (4/P-STAT3, against the phosphorylated Y705 of STAT3). Expression of the G-CSFR was measured with biotinylated G-CSF.28 Biotin conjugates were visualized by streptavidin-PerCP–Cy5.5 (BD Pharmingen), streptavidin-PE–Cy7, or streptavidin-PE. Where possible, cells were stained in the presence of anti–CD16/CD32 block (2.4G2) and dead cells were excluded by propidium iodide. Intracellular cytokine staining of stimulated T cells was performed as described previously.29 All Abs and secondary reagents were obtained from eBioscience unless otherwise specified. Flow cytometry analyses were performed on a FACSCanto (BD Biosciences) and data were analyzed using FlowJo Version 9.2 software (TreeStar).
Cell culture and stimulation
For liquid cultures, CMPs and GMPs were cultured for 3 days at 37°C in a humidified incubator at 5% CO2 in 96-well plates in X-VIVO-15 (Lonza) at a density of 1000 (CMPs) or 1500 (GMPs) cells per well in the presence of SCF and M-CSF or G-CSF. IFNγ was added to cultures when indicated. Concentrations used were: SCF, 5 ng/mL; G-CSF, 5 ng/mL; M-CSF, 5 ng/mL; and IFNγ, 20 ng/mL. CFSE staining of (100 000-150 000) progenitors was performed with 0.5μM CFSE (Invitrogen) in PBS for 12 minutes at 37°C. The number of cells in culture was quantified by flow cytometry and for CSFE histograms between 5000 and 20 000 cells measured. For colony-forming assays, 250 purified GMPs were plated in duplicate in methylcellulose medium (Methocult M3434; StemCell Technologies) in 6-well plates, supplemented with IFNγ when indicated, and colonies were scored at day 8. When cells were stimulated with IFNγ and/or G-CSF for pSTAT3 staining or RNA isolation, cells were starved for at least 1 hour in serum-free X-VIVO 15 medium before stimulation. Cytokines were obtained from Peprotech. For in vitro stimulation of BM T cells, total BM was stimulated for 5 hours with LCMV peptide GP33-41 (1 μg/mL, KAVYNFATC; Genscript) in the presence of brefeldin A (10 μg/mL; Sigma-Aldrich) in IMDM containing 10% FCS or with phorbol 12-myristate 13-acetate (PMA)/ionomycin as described previously.29
Quantitative real-time PCR
RNA extraction was done with TRIzol reagent (Invitrogen) and reverse transcribed to cDNA using random hexamers and Superscript II reverse transcriptase (Roche). Quantitative real-time PCR was performed in duplicate using Express SYBR Green ER (Invitrogen) on the StepOnePlus RT-PCR system (Applied Biosystems). Data were normalized using 18S rRNA as a reference gene. Primer sequences are available on request.
Adoptive transfer, LCMV infection, and anti-CD40 injection
T cells were isolated from lymph nodes and spleens using CD4 and CD8 microbeads (Miltenyi Biotec) and MACS-positive bead selection with LS columns (Miltenyi Biotec). Cells were resuspended in PBS and 10 × 106 cells were injected intravenously into CD27−/− and CD70TGxCD27−/− recipient mice, which were analyzed 3 weeks later. For LCMV infection, mice were infected intraperitoneally with 1 × 105 PFUs of LCMV clone Armstrong and analyzed at the indicated days. For sequential analysis, a drop of blood was collected by puncturing the vena saphena and analyzed by flow cytometry. An agonistic Ab to CD40 (100 μg, clone FGK-45) or a rat control Ab (100 μg, clone GL113) was injected intraperitoneally in 200 μL of PBS at days 1 and 3 and mice were analyzed at day 5.
BrdU
Bromodeoxyuridine (BrdU, 5 mg) was injected intraperitoneally in 200 μL of PBS. Collected cells were stained with the appropriate Abs and BrdU was visualized as described previously.30
pSTAT3 Phospho-Flow staining
32D cells expressing WT or mutant human G-CSFR31 were maintained in RPMI 1640 medium (Lonza) supplemented with 10% FCS and IL-3. When indicated, cells were pre-incubated with IFNγ for 45 minutes and subsequently stimulated with 5 ng/mL of murine G-CSF (for primary murine cells) or 10 ng/mL of human G-CSF28 (for 32D cells) for 30 minutes. Cells were fixed for 10 minutes at 37°C by adding an equal volume of BD Cytofix/Cytoperm buffer (BD Biosciences), chilled on ice for 1 minute, washed, and fixed with −20°C 90% methanol for a least 1 hour. Cells were washed and incubated with pSTAT3–Alexa Fluor 647 Ab at room temperature for 45 minutes.
Statistics
Mean values ± SD or SEM are shown. Statistical analysis was performed using either a paired or unpaired 2-tailed Student t test with GraphPad Prism 5 software.
Results
CD70TG mice have more monocytes and increased monopoiesis over granulopoiesis
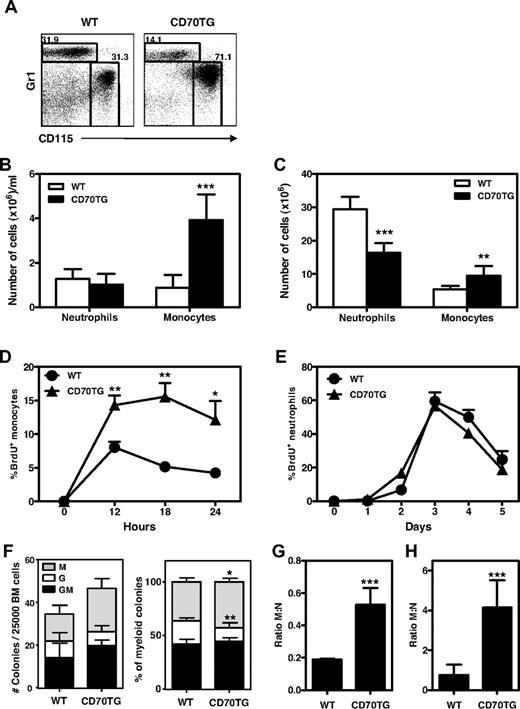
To examine the impact of T cell–driven immune activation on the development of monocytes and neutrophils, we analyzed the myeloid compartment of CD70TG mice because they have large numbers of effector T cells due to enhanced costimulation through CD27.23 We found that CD70TG mice contained relatively more monocytes (CD115+Gr1dim) than neutrophils (CD115−Gr1high) within the fraction of CD11b+ cells in the peripheral blood (Figure 1A). Quantification of these data revealed that CD70TG mice had a strong increase in the absolute numbers of circulating monocytes, whereas neutrophil numbers were not different from WT mice (Figure 1B). This increase in monocytes was also found in BM, suggesting that monocyte production was increased in CD70TG mice (Figure 1C). To test this, WT and CD70TG mice were injected with BrdU and the appearance of circulating monocytes that had incorporated BrdU was followed over time. We found more BrdU+ monocytes in blood from CD70TG than WT mice at 12, 18, and 24 hours after BrdU injection (Figure 1D). In contrast, the release of BrdU+ neutrophils was not altered (Figure 1E), which confirmed that the production of monocytes was increased in CD70TG mice.
CD70TG mice have increased numbers of monocytes and increased monopoiesis over granulopoiesis. (A) Representative plots of staining for monocytes (Gr1lowCD115+) and neutrophils (Gr1+CD115−) within the CD11b+ compartment in the peripheral blood of WT and CD70TG mice and absolute numbers of monocytes and neutrophils in the peripheral blood (B) and BM (C) of WT and CD70TG mice. Data in panels B and C represent means ± SD from at least 3 mice per group. (D-E) Percentage of BrdU+ monocytes within the CD11b+ compartment in the peripheral blood of WT and CD70TG mice measured 12, 18, and 24 hours after injection of BrdU (D) and percentage of BrdU+ neutrophils in the peripheral blood of WT and CD70TG mice measured at indicated days after BrdU injection (E). Data in panels D and E represent means ± SEM from 5 mice per group. (F) Type of colonies derived from total BM from WT and CD70TG mice cultured for 8 days in semisolid medium. Data are displayed as the absolute number of CFUs per 25 000 BM cells (left) or as percentage from total colonies (right). M indicates monocyte/macrophage; G, granulocyte; and GM, mixed monocyte/macrophage and granulocyte. Data represent means ± SD from 6 mice per group. M:N ratios in BM (G) and peripheral blood (H) of WT and CD70TG mice were measured by dividing the number of monocytes by the number of neutrophils. Data in panels G and H represent means ± SD from at least 3 mice per group. BM represents cell numbers per 2 femurs and 2 tibiae. All experiments were performed at least 2 times with similar results. *P < .05; **P < .01; ***P < .001.
CD70TG mice have increased numbers of monocytes and increased monopoiesis over granulopoiesis. (A) Representative plots of staining for monocytes (Gr1lowCD115+) and neutrophils (Gr1+CD115−) within the CD11b+ compartment in the peripheral blood of WT and CD70TG mice and absolute numbers of monocytes and neutrophils in the peripheral blood (B) and BM (C) of WT and CD70TG mice. Data in panels B and C represent means ± SD from at least 3 mice per group. (D-E) Percentage of BrdU+ monocytes within the CD11b+ compartment in the peripheral blood of WT and CD70TG mice measured 12, 18, and 24 hours after injection of BrdU (D) and percentage of BrdU+ neutrophils in the peripheral blood of WT and CD70TG mice measured at indicated days after BrdU injection (E). Data in panels D and E represent means ± SEM from 5 mice per group. (F) Type of colonies derived from total BM from WT and CD70TG mice cultured for 8 days in semisolid medium. Data are displayed as the absolute number of CFUs per 25 000 BM cells (left) or as percentage from total colonies (right). M indicates monocyte/macrophage; G, granulocyte; and GM, mixed monocyte/macrophage and granulocyte. Data represent means ± SD from 6 mice per group. M:N ratios in BM (G) and peripheral blood (H) of WT and CD70TG mice were measured by dividing the number of monocytes by the number of neutrophils. Data in panels G and H represent means ± SD from at least 3 mice per group. BM represents cell numbers per 2 femurs and 2 tibiae. All experiments were performed at least 2 times with similar results. *P < .05; **P < .01; ***P < .001.
Myeloid differentiation was further analyzed by measuring colony formation of total BM. These experiments revealed an increase of monocyte/macrophage colonies in CD70TG mice compared with WT mice and a small decrease in the contribution of neutrophil colonies (Figure 1F). Although higher in number, we found that the monocyte/macrophage colonies in CD70TG mice were smaller in size and thus contained fewer cells than the corresponding colonies in WT mice (data not shown). Finally, to better visualize the balance between monocyte and neutrophil output in vivo, we calculated the ratio between monocytes and neutrophils (the M:N ratio), which was increased in both the BM (Figure 1G) and blood (Figure 1H) of CD70TG mice. These data demonstrate that CD27-driven immune activation differentially affects myelopoiesis, shifting the formation of myeloid cells toward the monocyte lineage.
IFNγ induces monopoiesis over granulopoiesis in vivo and in vitro
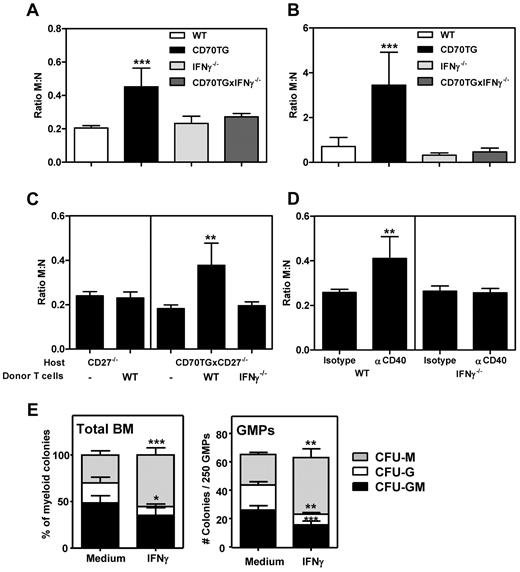
Because CD70TG mice have increased numbers of IFNγ-producing T cells in the BM,20,23 we investigated whether the increased monopoiesis over neutrophil development in CD70TG mice was dependent on IFNγ. We found that the increased M:N ratio in CD70TG mice was restored to WT levels when these mice were backcrossed on an IFNγ-deficient background, both in the BM (Figure 2A) and blood (Figure 2B), demonstrating that IFNγ is indeed responsible for the observed skewing of monocytes over neutrophils in this model. To determine whether T cell–derived IFNγ is also sufficient for altering the balance between monocyte and neutrophil formation, we used a previously described adoptive transfer model of T cells to CD27-deficient CD70TG mice.23 CD27−/− and CD70TGxCD27−/− mice have normal myeloid differentiation, and transfer of T cells into these mice results in CD70-dependent activation and accumulation of IFNγ-producing effector T cells in BM.20,23 Transfer of WT T cells to CD70TGxCD27−/− mice increased the M:N ratio, which was completely dependent on IFNγ production by these transferred T cells (Figure 2C). To test the effect of IFNγ on the M:N ratio in a T-cell activation model that is not driven by transgenic CD70 expression, we injected WT and IFNγ−/− mice with an agonistic anti-CD40 Ab, which increases the number of IFNγ-producing T cells.20,32 We found that the M:N ratio increased in the BM of WT mice injected with anti-CD40, but not in IFNγ−/− mice (Figure 2D). In both experimental settings, we observed the same pattern of M:N ratio in the peripheral blood (data not shown). Finally, because both monocytes and neutrophils are derived from GMPs and because we have previously shown that GMPs express the IFNγ-receptor,20 we investigated whether IFNγ directly affects monocyte versus neutrophil differentiation from GMPs. We found that the addition of IFNγ to WT GMPs did indeed increase the formation of monocyte/macrophage colonies at the cost of neutrophil colonies (Figure 2E). These experiments demonstrate that IFNγ is sufficient to enhance monocyte over neutrophil development both in vitro and in vivo and that this effect can be mediated at the level of the GMP.
IFNγ induces monopoiesis over granulopoiesis in vivo and in vitro. M:N ratios in the BM (A) and peripheral blood (B) of WT, CD70TG, IFNγ−/−, and CD70TGxIFNγ−/− mice were measured by dividing the number of monocytes by the number of neutrophils. Data in panels A and B represent means ± SD from at least 3 mice per group. (C) M:N ratios in BM of CD27−/− and CD70TGxCD27−/− control mice and 3 weeks after transfer of WT or IFNγ−/− T cells. (D) M:N ratios in BM of WT and IFNγ−/− mice 4 days after the first injection of αCD40 or control IgG. Data in panels C and D represent means ± SD from 5 mice per group. (E) Type of colonies derived from total WT BM (left; means ± SD from 6 mice per group) or from 250 purified GMPs (right; means ± SD from 4 mice per group) cultured for 8 days in semisolid medium with or without IFNγ. M indicates monocyte/macrophage; G, granulocyte; and GM, mixed monocyte/macrophage and granulocyte. All experiments were performed at least 2 times with similar results. *P < .05; **P < .01; ***P < .001.
IFNγ induces monopoiesis over granulopoiesis in vivo and in vitro. M:N ratios in the BM (A) and peripheral blood (B) of WT, CD70TG, IFNγ−/−, and CD70TGxIFNγ−/− mice were measured by dividing the number of monocytes by the number of neutrophils. Data in panels A and B represent means ± SD from at least 3 mice per group. (C) M:N ratios in BM of CD27−/− and CD70TGxCD27−/− control mice and 3 weeks after transfer of WT or IFNγ−/− T cells. (D) M:N ratios in BM of WT and IFNγ−/− mice 4 days after the first injection of αCD40 or control IgG. Data in panels C and D represent means ± SD from 5 mice per group. (E) Type of colonies derived from total WT BM (left; means ± SD from 6 mice per group) or from 250 purified GMPs (right; means ± SD from 4 mice per group) cultured for 8 days in semisolid medium with or without IFNγ. M indicates monocyte/macrophage; G, granulocyte; and GM, mixed monocyte/macrophage and granulocyte. All experiments were performed at least 2 times with similar results. *P < .05; **P < .01; ***P < .001.
IFNγ is required to increase monocyte numbers and suppress neutrophilia during LCMV infection
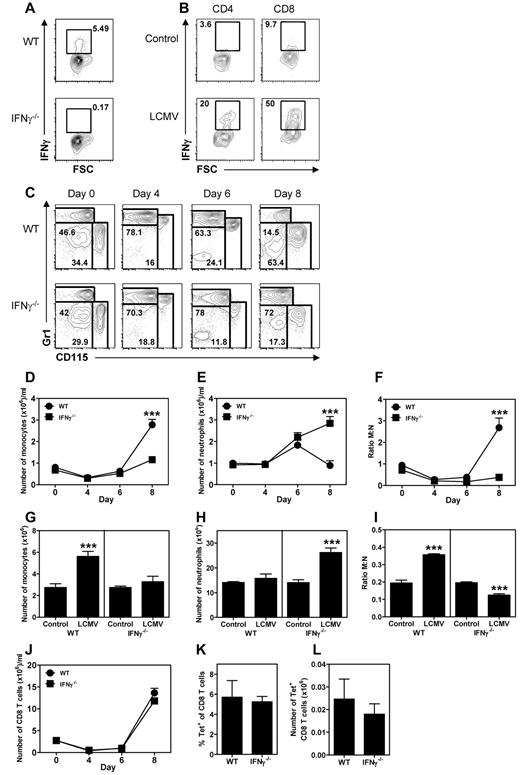
To determine whether IFNγ also modulates the development of monocytes and neutrophils during a viral infection, WT and IFNγ−/− mice were infected with LCMV Armstrong. Infection with this virus results in a type I IFN-dependent leukopenia early after infection, whereas IFNγ-producing T cells can only be found 7-8 days after the onset of infection.33 It was previously shown that IFNγ is not required for a proper adaptive immune response against LCMV or for viral clearance.33 After LCMV infection, virus-specific T cells can be found in the BM, which produce IFNγ after stimulation with LCMV peptide (Figure 3A) or PMA/ionomycin (Figure 3B), indicating that locally produced IFNγ could affect myelopoiesis in BM. WT and IFNγ−/− mice responded similarly to the leukopenia during the first days after infection, showing a reduction in the numbers of circulating monocytes but not neutrophils (Figure 3C-E), thereby reducing the M:N ratio during the first days after infection (Figure 3F). However, at day 8 of the infection, monocyte numbers strongly increased in the peripheral blood of WT mice, but not in IFNγ−/− mice (Figure 3D). In contrast, neutrophil numbers had returned to baseline levels in WT mice at day 8 (Figure 3D), but were significantly increased in IFNγ−/− mice (Figure 3E). In accordance with these findings in the peripheral blood, monocyte numbers were increased in the BM of WT mice 8 days after infection (Figure 3G), whereas neutrophil numbers were increased in IFNγ−/− mice (Figure 3H), resulting in an increased M:N ratio in BM of WT mice and a decreased M:N ratio in IFNγ−/− mice (Figure 3I). Remarkably, the observed changes in circulating monocyte and neutrophil numbers between WT and IFNγ−/− mice were found at day 8, which corresponds with the peak of the antiviral T-cell response (Figure 3J). IFNγ deficiency did not affect the kinetics and magnitude (Figure 3J) nor the specificity (Figure 3K) of the T-cell response. In addition, equal numbers of LCMV-specific T cells were found in the BM of WT and IFNγ−/− mice (Figure 3L), suggesting that the distinct IFNγ-dependent effects on myelopoiesis do not result from an altered T-cell response. These experiments demonstrate that IFNγ induces an expansion of the monocyte compartment and is required to suppress an increase in neutrophils during LCMV infection.
IFNγ is required to increase monocyte numbers and suppress neutrophilia during LCMV infection. Representative plot of intracellular staining for IFNγ in T cells from BM of WT and IFNγ−/− mice stimulated in vitro 8 days after LCMV infection with LCMV peptide (A) or PMA/ionomycin (B). (C) Representative plot of staining for monocytes (Gr1lowCD115+) and neutrophils (Gr1+CD115−) in the peripheral blood before LCMV infection and at the indicated days after infection. Numbers of monocytes (D) and neutrophils (E) in the peripheral blood of WT and IFNγ−/− mice at the indicated days after LCMV infection and M:N ratios in the peripheral blood of these mice (F) are shown. Data in panels D through F represent means ± SEM from 5 mice per group. Numbers of monocytes (G) and neutrophils (H) in the BM of WT and IFNγ−/− naive mice and LCMV-infected mice 8 days after infection and the M:N ratio in the BM of these mice (I) are shown. Data in panels G through I represent means ± SD from 5 mice per group; BM represents cell numbers per 2 femurs and 2 tibiae. (J) Number of CD8 T cells in the peripheral blood of WT and IFNγ−/− mice at the indicated days after LCMV infection. (K-L) Percentage of tetramer-binding cells of total CD8 T cells in the peripheral blood (K) and number of tetramer-binding CD8 T cells in BM of WT and IFNγ−/− mice 8 days after LCMV infection (L). Data in panel J represent means ± SEM from 5 mice per group and data in panels K and L represent means ± SD from 5 mice per group. All data shown are representative of at least 2 independent experiments. *P < .05; **P < .01; ***P < .001.
IFNγ is required to increase monocyte numbers and suppress neutrophilia during LCMV infection. Representative plot of intracellular staining for IFNγ in T cells from BM of WT and IFNγ−/− mice stimulated in vitro 8 days after LCMV infection with LCMV peptide (A) or PMA/ionomycin (B). (C) Representative plot of staining for monocytes (Gr1lowCD115+) and neutrophils (Gr1+CD115−) in the peripheral blood before LCMV infection and at the indicated days after infection. Numbers of monocytes (D) and neutrophils (E) in the peripheral blood of WT and IFNγ−/− mice at the indicated days after LCMV infection and M:N ratios in the peripheral blood of these mice (F) are shown. Data in panels D through F represent means ± SEM from 5 mice per group. Numbers of monocytes (G) and neutrophils (H) in the BM of WT and IFNγ−/− naive mice and LCMV-infected mice 8 days after infection and the M:N ratio in the BM of these mice (I) are shown. Data in panels G through I represent means ± SD from 5 mice per group; BM represents cell numbers per 2 femurs and 2 tibiae. (J) Number of CD8 T cells in the peripheral blood of WT and IFNγ−/− mice at the indicated days after LCMV infection. (K-L) Percentage of tetramer-binding cells of total CD8 T cells in the peripheral blood (K) and number of tetramer-binding CD8 T cells in BM of WT and IFNγ−/− mice 8 days after LCMV infection (L). Data in panel J represent means ± SEM from 5 mice per group and data in panels K and L represent means ± SD from 5 mice per group. All data shown are representative of at least 2 independent experiments. *P < .05; **P < .01; ***P < .001.
IFNγ directs myelopoiesis during LCMV infection
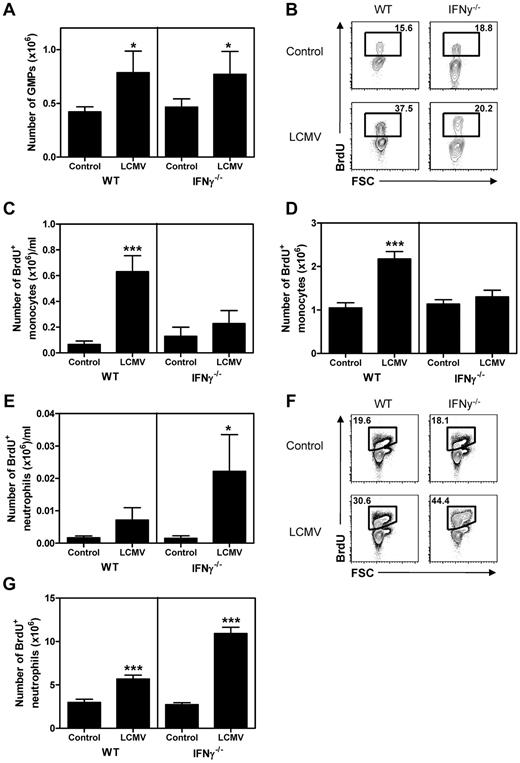
To investigate whether the IFNγ-induced changes in monocyte and neutrophil numbers on LCMV infection were indeed due to corresponding changes in myelopoiesis, we examined myeloid progenitors and the production of new progeny. Analysis of GMPs by flow cytometry revealed that the absolute number of these myeloid-committed progenitors was increased in LCMV-infected mice independently of IFNγ (Figure 4A). However, BrdU incorporation experiments demonstrated a strong increase of BrdU+ monocytes in the blood (Figure 4B-C) and BM (Figure 4D) of WT mice at day 8 of infection, but not in IFNγ−/− mice. Although very low numbers of circulating BrdU+ neutrophils could be detected in naive mice or WT LCMV-infected mice, a strong increase in BrdU+ neutrophils was observed in the blood of IFNγ−/− infected mice (Figure 4E). Correspondingly, we found a much stronger increase of BrdU+ neutrophils in the BM of IFNγ−/− than WT mice after infection (Figure 4F-G). These data demonstrate that the increase of monocytes in LCMV-infected WT mice and neutrophils in IFNγ−/− mice is indeed the direct result of increased production of these cells in BM and that IFNγ is able to redirect myelopoiesis toward the monocytic lineage during viral infection.
IFNγ directs myelopoiesis during LCMV infection. (A) Number of GMPs in BM of WT and IFNγ−/− naive mice and 8 days after LCMV infection. (B) Representative plot of BrdU staining in monocytes in the peripheral blood of naive and LCMV-infected mice 8 days after infection and absolute numbers of BrdU+ monocytes in peripheral blood (C) and BM (D) of these mice. (E) Number of BrdU+ neutrophils in the peripheral blood of naive and LCMV-infected mice 8 days after infection. (F-G) Representative plot of BrdU staining in neutrophils in BM (F) and number of BrdU+ neutrophils in BM of these mice (G) are shown. Data in panels A, C, D, and E represent means ± SD from 5 mice per group; BM represents cell numbers per 2 femurs and 2 tibiae. Experiments were performed at least 2 times with similar results. *P < .05; **P < .01; ***P < .001.
IFNγ directs myelopoiesis during LCMV infection. (A) Number of GMPs in BM of WT and IFNγ−/− naive mice and 8 days after LCMV infection. (B) Representative plot of BrdU staining in monocytes in the peripheral blood of naive and LCMV-infected mice 8 days after infection and absolute numbers of BrdU+ monocytes in peripheral blood (C) and BM (D) of these mice. (E) Number of BrdU+ neutrophils in the peripheral blood of naive and LCMV-infected mice 8 days after infection. (F-G) Representative plot of BrdU staining in neutrophils in BM (F) and number of BrdU+ neutrophils in BM of these mice (G) are shown. Data in panels A, C, D, and E represent means ± SD from 5 mice per group; BM represents cell numbers per 2 femurs and 2 tibiae. Experiments were performed at least 2 times with similar results. *P < .05; **P < .01; ***P < .001.
IFNγ impairs the proliferation and differentiation of myeloid progenitors in response to G-CSF
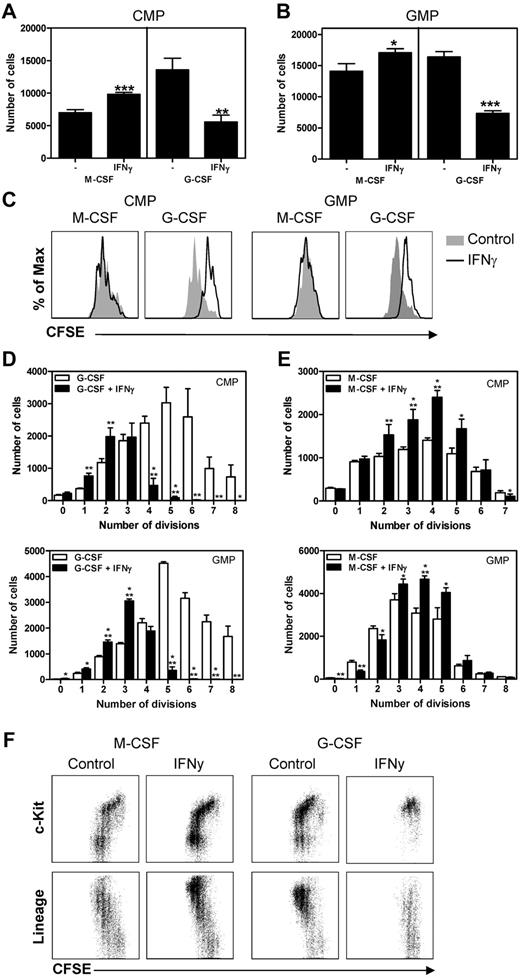
Up to this point, we have shown that IFNγ induces the production of monocytes, whereas it negatively affects neutrophil development. Monocyte and neutrophil production from hematopoietic progenitors is induced by M-CSF and G-CSF, respectively.6 To determine whether IFNγ directs monocyte and neutrophil differentiation by affecting cytokine responses, purified CMPs and GMPs were cultured for 3 days in the presence of M-CSF or G-CSF with or without IFNγ. We found that IFNγ slightly increased the outgrowth of CMPs (Figure 5A) and GMPs (Figure 5B) in response to M-CSF, whereas IFNγ strongly reduced the outgrowth of these cells in response to G-CSF; these effects could not be explained by corresponding changes in cell death (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). We investigated whether IFNγ impairs the proliferative response to G-CSF by labeling purified progenitors with CFSE and subsequently culturing these cells as described in Figure 5A and B. Indeed, proliferation of CMPs and GMPs in response to G-CSF was significantly reduced by IFNγ (Figure 5C-D). In contrast, IFNγ did not negatively affect—and even slightly enhanced—the proliferative response to M-CSF (Figure 5E). After differentiation of progenitor cells, expression of c-Kit is gradually lost and expression of mature lineage markers is increased. Flow cytometric analysis of cultured CFSE-labeled cells indeed revealed that IFNγ impaired G-CSF–induced differentiation of progenitors, because the cells in culture were mainly c-Kit+ and did not express lineage markers to the same extent as the cells cultured without IFNγ (Figure 5F). These experiments demonstrate that IFNγ directly inhibits the proliferation and differentiation of myeloid progenitors in response to G-CSF while it promotes M-CSF–induced proliferation/differentiation.
IFNγ impairs the proliferation and differentiation of myeloid progenitors in response to G-CSF. Absolute cell numbers derived from 1000 CMPs (A) and 1500 GMPs (B) after 3 days of culture with M-CSF or G-CSF with or without IFNγ. (C) Histograms showing CFSE dilution of CMPs and GMPs cultured for 3 days with M-CSF or G-CSF with or without IFNγ and absolute number of cells in each CFSE dilution peak of G-CSF–cultured (D) and M-CSF–cultured (E) CMPs and GMPs. (F) Representative plots showing CFSE dilution versus c-Kit or lineage expression of CFSE-labeled CMPs cultured for 3 days with M-CSF or G-CSF with or without IFNγ. Data in panels A, B, and D represent means ± SD from triplicate cultures and all data are representative of 3 independent experiments. *P < .05; **P < .01; ***P < .001.
IFNγ impairs the proliferation and differentiation of myeloid progenitors in response to G-CSF. Absolute cell numbers derived from 1000 CMPs (A) and 1500 GMPs (B) after 3 days of culture with M-CSF or G-CSF with or without IFNγ. (C) Histograms showing CFSE dilution of CMPs and GMPs cultured for 3 days with M-CSF or G-CSF with or without IFNγ and absolute number of cells in each CFSE dilution peak of G-CSF–cultured (D) and M-CSF–cultured (E) CMPs and GMPs. (F) Representative plots showing CFSE dilution versus c-Kit or lineage expression of CFSE-labeled CMPs cultured for 3 days with M-CSF or G-CSF with or without IFNγ. Data in panels A, B, and D represent means ± SD from triplicate cultures and all data are representative of 3 independent experiments. *P < .05; **P < .01; ***P < .001.
IFNγ induces the expression of IRF8 and PU.1
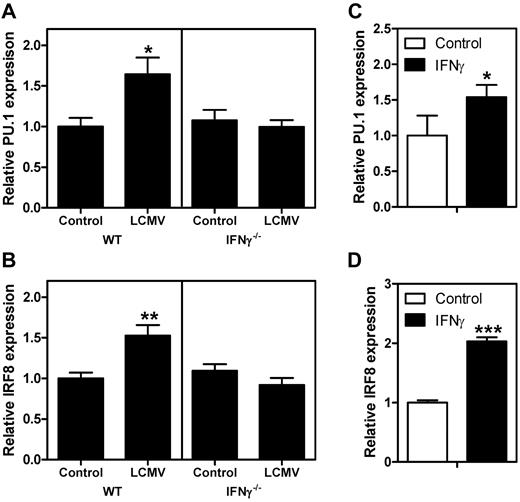
Induction of monopoiesis is mainly controlled by the transcription factors PU.1 and IRF8.12 IRF8 is directly inducible by IFNγ,34 and we have previously reported that IFNγ increases PU.1 expression in primary progenitor cells in an IRF1-dependent manner.24 To determine whether the observed monopoiesis-promoting effect of IFNγ on hematopoietic progenitors was related to increased expression of PU.1 and IRF8, mRNA levels were measured in GMPs from naive and LCMV-infected mice. Both PU.1 (Figure 6A) and IRF8 (Figure 6B) mRNA levels were elevated in WT infected mice, but were unaffected in IFNγ−/− infected mice. To determine whether IFNγ directly affects the expression of these factors, purified GMPs were stimulated with IFNγ and mRNA levels of PU.1 and IRF8 were measured. We found that IFNγ increased the expression of both PU.1 (Figure 6C) and IRF8 (Figure 6D). These observations demonstrate that IFNγ is sufficient to elevate the expression of these monopoiesis-inducing transcription factors in GMPs both in vitro and in vivo.
IFNγ induces the expression of IRF8 and PU.1. Quantitative PCR analysis of expression levels of PU.1 (A) and IRF8 (B) in purified GMPs from naive mice and from mice 8 days after LCMV infection. Expression is relative to the levels in naive WT GMPs. Relative expression of PU.1 (C) and IRF8 (D) in WT GMPs after in vitro stimulation with IFNγ. Expression is relative to the levels in unstimulated GMPs. Data in panels A and B represent means ± SD from 3 independent experiments with 2 mice per group; data in panels C and D represent means ± SD from triplicate stimulations measured in duplicate. *P < .05; **P < .01; ***P < .001.
IFNγ induces the expression of IRF8 and PU.1. Quantitative PCR analysis of expression levels of PU.1 (A) and IRF8 (B) in purified GMPs from naive mice and from mice 8 days after LCMV infection. Expression is relative to the levels in naive WT GMPs. Relative expression of PU.1 (C) and IRF8 (D) in WT GMPs after in vitro stimulation with IFNγ. Expression is relative to the levels in unstimulated GMPs. Data in panels A and B represent means ± SD from 3 independent experiments with 2 mice per group; data in panels C and D represent means ± SD from triplicate stimulations measured in duplicate. *P < .05; **P < .01; ***P < .001.
IFNγ reduces G-CSF–mediated STAT3 phosphorylation by a SOCS3-dependent mechanism
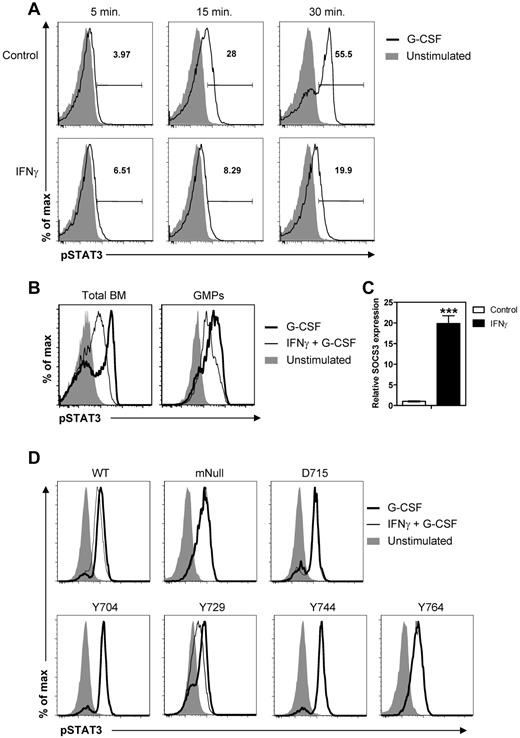
The observed IFNγ-mediated reduction in the responsiveness of progenitors to G-CSF could have resulted from reduced expression of the G-CSFR. However, IFNγ did not affect the expression of G-CSFR on progenitors in CD70TG mice, LCMV-infected mice, or cultured progenitors (data not shown). Therefore, we examined whether signaling through the G-CSFR was altered by IFNγ. G-CSFR signaling induces phosphorylation of STAT3, which induces expression of the transcription factors required for expansion and differentiation of progenitor cells toward the neutrophil lineage. To determine whether IFNγ reduced the phosphorylation of STAT3 in response to G-CSF, total BM cells were cultured for 5, 15, or 30 minutes with G-CSF with or without a 45-minute preincubation with IFNγ, and the levels of phosphorylated STAT3 (pSTAT3) were measured by flow cytometry. Indeed, we found that G-CSF induced a time-dependent increase in phosphorylation of STAT3, which was strongly inhibited by IFNγ (Figure 7A). IFNγ also reduced the G-CSF–induced phosphorylation of STAT3 in purified GMPs (Figure 7B).
IFNγ reduces G-CSF–mediated STAT3 phosphorylation in a SOCS3-dependent manner. (A) Representative histograms showing intracellular staining for pSTAT3 in total BM cells stimulated for the indicated times with G-CSF with or without 45 minutes of preincubation with IFNγ. (B) Representative histograms showing overlays of intracellular staining for pSTAT3 in total BM cells and GMPs after 30 minutes of stimulation with G-CSF with or without preincubation with IFNγ. (C) Relative expression of SOCS3 in WT GMPs after 30 minutes in vitro stimulation with IFNγ measured by quantitative PCR analysis. Expression is relative to the levels in unstimulated GMPs. Data represent means ± SD from triplicate cultures. (D) Representative histograms showing overlays of intracellular staining for pSTAT3 in 32D cells expressing WT or a mutant human G-CSFR after 30 minutes of stimulation with G-CSF with or without preincubation with IFNγ. The SOCS3-recruiting tyrosine motif Y729 is only present in cells expressing WT G-CSFR or the mutant receptor denoted as Y729. All data are representative of at least 2 independent experiments. ***P < .001.
IFNγ reduces G-CSF–mediated STAT3 phosphorylation in a SOCS3-dependent manner. (A) Representative histograms showing intracellular staining for pSTAT3 in total BM cells stimulated for the indicated times with G-CSF with or without 45 minutes of preincubation with IFNγ. (B) Representative histograms showing overlays of intracellular staining for pSTAT3 in total BM cells and GMPs after 30 minutes of stimulation with G-CSF with or without preincubation with IFNγ. (C) Relative expression of SOCS3 in WT GMPs after 30 minutes in vitro stimulation with IFNγ measured by quantitative PCR analysis. Expression is relative to the levels in unstimulated GMPs. Data represent means ± SD from triplicate cultures. (D) Representative histograms showing overlays of intracellular staining for pSTAT3 in 32D cells expressing WT or a mutant human G-CSFR after 30 minutes of stimulation with G-CSF with or without preincubation with IFNγ. The SOCS3-recruiting tyrosine motif Y729 is only present in cells expressing WT G-CSFR or the mutant receptor denoted as Y729. All data are representative of at least 2 independent experiments. ***P < .001.
G-CSF induces STAT3-dependent expression of SOCS3,35 which serves as a negative regulator of G-CSFR signaling by reducing the phosphorylation of STAT3.36 IFNγ is known to induce the expression of multiple SOCS family members in a variety of cell types.37 To determine whether IFNγ increases SOCS3 expression in myeloid progenitors, GMPs were purified and stimulated with recombinant IFNγ for 30 minutes. We found that SOCS3 mRNA levels in GMPs increased almost 20-fold after stimulation with IFNγ (Figure 7C). To determine whether the impaired phosphorylation of STAT3 is indeed dependent on the IFNγ-induced expression of SOCS3, the effect of IFNγ on G-CSF–mediated STAT3 phosphorylation was measured in the murine myeloid progenitor cell line 32D expressing WT or mutant human G-CSFR.31 The cytoplasmic tail of the human G-CSFR contains 4 conserved tyrosine-based motifs (with tyrosine residues at positions 704, 729, 744, and 764), which are involved in recruiting factors regulating G-CSFR signaling. Y729 has been shown to be the recruitment site for SOCS3, and a mutant G-CSFR lacking Y729 is insensitive to SOCS3-mediated inhibition of G-CSF–induced signaling.28,38 We compared pSTAT3 levels in cells expressing the WT G-CSFR with a mutant in which all 4 tyrosine motifs were substituted with phenylalanine (mNull), a truncated mutant lacking that part of the cytoplasmic tail of the G-CSFR that contains Y729, Y744, and Y764 (D715), and 4 different mutants in which only 1 tyrosine motif was intact and the other 3 were replaced with phenylalanine (Y704, Y729, Y744, and Y764). Although less pronounced compared with primary BM cells, IFNγ significantly reduced pSTAT3 levels in G-CSF–stimulated 32D cells expressing WT G-CSFR (Figure 7D; GeoMFI, mean ± SD, G-CSF: 1146 ± 0.7 vs IFNγ + G-CSF: 963 ± 21.2, P < .01). IFNγ did not affect STAT3 phosphorylation when all 4 G-CSFR tyrosine motifs were substituted (mNull) in the truncated mutant (D715) nor when only Y704, Y744, or Y764 was present (Figure 7D). However, IFNγ did significantly reduce pSTAT3 levels in cells that still expressed the SOCS3-recruiting motif Y729 (Figure 7D; GeoMFI, mean ± SD, G-CSF: 484 ± 1.7 vs IFNγ + G-CSF: 373 ± 13.2, P < .001). These experiments demonstrate that IFNγ impairs G-CSF–induced STAT3 phosphorylation, which is dependent on the recruitment of IFNγ-induced SOCS3 to tyrosine motif Y729 of the G-CSFR.
Discussion
Multiple factors modulate hematopoiesis during stress responses such as infection and inflammation to mount a proper immune response to the particular type of invading pathogen. Previously, effects of IFNγ on HSC self-renewal,39 B-cell lymphopoiesis,23 eosinophil development,20 and erythropoiesis24 have been reported, suggesting that IFNγ modulates hematopoiesis at multiple levels. In the present study, we have demonstrated that IFNγ is also an important factor in modulating the differentiation of myeloid progenitors into neutrophils and monocytes. Using various mouse models and in vitro systems, we show that IFNγ directly induces monopoiesis over neutrophil development. Moreover, IFNγ is required to induce monopoiesis and suppress neutrophil development during acute viral infection with LCMV. Previous studies have demonstrated severe neutrophilia after infection of IFNγ−/− mice with various pathogens25-27 and an inhibiting effect of IFNγ on granulocyte colony formation from human progenitor cells in vitro,40 supporting our finding that IFNγ suppresses neutrophil production. Similar to viral infection, infection with intracellular bacteria induces profound IFNγ production, which might explain the observed increase in monocyte numbers after infection with such pathogens.13,25 A recent study demonstrated that IFNγ signaling is also required to increase the frequency of monocytes in circulation after infection with the intracellular bacterium Ehrlichia muris, confirming the important role of IFNγ in inducing monopoiesis.25 Whereas IFNγ is required for the control of persistent LCMV infection, it is not required for clearance of LCMV clone Armstrong during acute infection,41 excluding the confounding effects of viral infection in IFNγ−/− mice in our experiments. During LCMV infection, T cells have been shown to enter the BM17 and inhibit both granulopoiesis and erythropoiesis through IFNγ production.21 Based on our current and previous findings,20,23,24 we postulate that IFNγ-producing T cells adjust the hematopoietic process during viral infection to improve the antiviral response. These findings have important clinical relevance, because IFNγ-producing T cells have been associated with BM failure in patients with aplastic anemia,42 suggesting that transient production of IFNγ is required to modulate hematopoiesis during infection, whereas prolonged production of IFNγ results in the suppression of multiple hematopoietic lineages and anemia.43
The observed effects of IFNγ on differentiation of GMPs in vitro and in LCMV-infected WT mice demonstrate that IFNγ promotes monopoiesis and suppresses neutrophilia. We found that IFNγ elevates the mRNA expression levels of IRF8 and PU.1 in GMPs. IRF8 is an important regulator in neutrophil versus monocyte development. IRF8-deficient mice display an increase in neutrophils and IRF8-deficient progenitors have an impaired macrophage differentiation.44,45 Moreover, forced expression of IRF8 in IRF8-deficient myeloid progenitor cells induces differentiation of these cells into macrophages and represses granulocyte-specific genes and G-CSF–mediated differentiation into granulocytes.46 PU.1 is well known as the master regulator in myeloid cell development.12 Low PU.1 levels induce granulopoiesis, whereas higher PU.1 levels induce monopoiesis.47 Phosphorylation of PU.1 allows interaction with IRF8,48 and increased macrophage development in zebrafish resulting from overexpression of IRF8 is dependent on the presence of PU.1.49 Finally, we have recently shown that IFNγ induces an IRF-1–mediated up-regulation of PU.1 in CMPs, which causes a decrease in erythroid and an increase in myeloid output.24 Therefore, the stimulating effect of IFNγ on monocyte development can be explained by an increase of PU.1 and IRF-8 in myeloid progenitor cells. The IFNγ-induced increase in expression of these transcription factors likely predisposes myeloid progenitors for monocyte differentiation, because the increased CFU-M capacity of total BM from CD70TG mice is maintained in vitro in the absence of exogenous IFNγ.
In addition to the positive impact of IFNγ on monocyte-inducing factors such as PU.1 and IRF-8, IFNγ inhibits G-CSF–mediated neutrophil differentiation. G-CSF induces the proliferation, differentiation, and survival of progenitor cells and neutrophils, and elevated levels of G-CSF are involved in emergency granulopoiesis.10,50 G-CSF–mediated phosphorylation of STAT3 induces expression of CCAAT/enhancer-binding protein β and accelerates cell-cycle progression and maturation of neutrophils during emergency responses.11,50 STAT3-induced expression of SOCS3 serves as a negative feedback loop by interfering with the phosphorylation of STAT3.35,36 In agreement with this, SOCS3-deficient cells display prolonged STAT3 activation and are hyperresponsive to G-CSF. Injection of G-CSF into SOCS3-deficient mice results in peripheral neutrophilia and inflammatory infiltration of neutrophils in various tissues, demonstrating that SOCS3 is also required to suppress G-CSF–induced emergency granulopoiesis.36 Recruitment of SOCS3 to tyrosine 729 of the cytoplasmic tail of the G-CSFR is essential for the inhibiting effect of SOCS3 on STAT signaling.38 Our experiments demonstrate that IFNγ induces SOCS3 expression in GMPs and that the observed IFNγ-mediated reduction in G-CSF–dependent STAT3 phosphorylation is dependent on SOCS3 recruitment to Y729. These observations support the hypothesis that IFNγ acts both as a promoter of monopoiesis and as a suppressor of neutrophil development.
Infection with intracellular viral and bacterial pathogens induces the production of IFNγ, and both type of infections result in increased monocyte production. It is tempting to speculate that the IFNγ-dependent mechanisms underlying the induction of monopoiesis and suppression of neutrophil production are similar in both types of intracellular infections. Macrophages, derived from BM monocytes, are crucial in controlling infection with intracellular pathogens and Ag-presenting, monocyte-derived dendritic cells contribute to shaping adaptive immunity against these pathogens.51 Therefore, there is a strong requirement to induce monocyte production over neutrophil development during intracellular pathogenic challenges. It has been suggested previously that neutrophils found at sites of viral infection contribute to the suppression of viral replication.52,53 However, in these studies, characterization of neutrophils ex vivo and depletion of neutrophils in vivo was performed using an Ab against the neutrophil marker Gr1 (clone RB6-8C5). Although the neutrophil-specific surface molecule Ly6G is the major Ag detected by this Ab, Gr1-Ab also binds to the Ly6C Ag, which is moderately expressed on monocytes during homeostasis and the expression of which increases significantly after infection (Figure 3C). Therefore, injection of Gr1-Abs in mice depletes neutrophils and monocytes and other Ly6C-expressing leukocytes, particularly during infections, which conflicts with the conclusions of previous findings on the contribution of Gr1-characterized or depleted neutrophils in controlling viral infection.52,53 Injection of the neutrophil-specific Ly6G Ab (1A8) in mice depletes neutrophils but not Gr1+ monocytes, making these Abs a useful tool in studies on the effect of neutrophil depletion during viral infection.54 Indeed, depletion of neutrophils with the Ly6G-specific Ab had no effect on the replication of HSV-1, whereas depletion with the Gr1-Ab exacerbated virus replication; this suggests an important role for monocytes in controlling this viral infection.55 However, another recent study reported increased replication of influenza virus after depletion of neutrophils with the Ly6G-Ab, suggesting that the contribution of neutrophils in controlling viral infection may also depend on the type and/or site of viral infection.56
In the present study, we have demonstrated that IFNγ is an important cytokine in directing myelopoiesis during acute viral infection. IFNγ directly stimulates monopoiesis by inducing the expression of lineage-specific regulators of myelopoiesis and suppresses neutrophil production by perturbing G-CSF–mediated signaling.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Cláudia Brandão Silva for technical assistance, B. Hooibrink for cell sorting, the staff of the animal facility of the AMC for excellent animal care, and Prof Dr René van Lier for stimulating discussion and critical reading of the manuscript.
This work was supported by a VIDI grant from The Netherlands Organization of Scientific Research (M.A.N.; 917.76.310) and a project grant from the Landsteiner Foundation for Blood Transfusion Research (M.A.N.; 0607).
Authorship
Contribution: A.M.d.B. designed and performed the experiments, analyzed the data, and wrote the manuscript; S.F.L. and M.V. performed the experiments and analyzed the data; L.B. provided the reagents; I.P.T. designed the experiments; and M.A.N. designed the experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Martijn A. Nolte, Department of Hematopoiesis, Sanquin Research and Landsteiner Laboratory, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: m.nolte@sanquin.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal