Abstract
Effector CD8+ T cells are believed to be terminally differentiated cells having cytotoxic activity and the ability to produce effector cytokines such as INF-γ and TNF-α. We investigated the difference between CXCR1+ and CXCR1− subsets of human effector CD27−CD28−CD8+ T cells. The subsets expressed cytolytic molecules similarly and exerted substantial cytolytic activity, whereas only the CXCR1− subset had IL-2 productivity and self-proliferative activity and was more resistant to cell death than the CXCR1+ subset. These differences were explained by the specific up-regulation of CAMK4, SPRY2, and IL-7R in the CXCR1− subset and that of pro-apoptotic death-associated protein kinase 1 (DAPK1) in the CXCR1+ subset. The IL-2 producers were more frequently found in the IL-7R+ subset of the CXCR1− effector CD8+ T cells than in the IL-7R− subset. IL-7/IL-7R signaling promoted cell survival only in the CXCR1− subset. The present study has highlighted a novel subset of effector CD8+ T cells producing IL-2 and suggests the importance of this subset in the homeostasis of effector CD8+ T cells.
Introduction
In a primary viral infection, naive CD8+ T cells are primed in secondary lymph nodes and consequently proliferate and differentiate into effector CD8+ T cells to eliminate virus-infected cells.1 After the viral clearance, most effector CD8+ T cells contract because of the apoptosis that occurs during the contraction phase,2 but a small number of these CD8+ T cells form a memory T-cell pool.3
In the mouse model of lymphocytic choriomeningitis virus (LCMV) infection, LCMV-specific CD8+ T cells rapidly expand and differentiate into effector CD8+ T cells for 6-8 days after the infection in response to Ags, resulting in viral clearance.3-5 During this maturation process, naive CD8+ T cells lose the expression of costimulatory molecules CD27, CD28, and IL-7 receptor α chain (IL-7Rα); at the same time, these cells express cytolytic effector molecules such as granzyme B and natural killer cell receptor, KLRG-1 (killer cell lectin-like receptor G1) and differentiate into effector CD8+ T cells.5,6 Perforin-deficient mice, which do not have functional effector CD8+ T cells, are unable to eliminate the LCMV and die within 10 days after infection.7 These and other studies indicate that effector CD8+ T cells directly play a crucial role in viral elimination.
Previous studies demonstrated that human CD8+ T cells undergo a change in the expression of costimulatory molecules such as CD27, CD28, and CD45RA on their surfaces according to their differentiation and maturation.8,9 These molecules have been used for phenotypic classification of human CD8+ T cells. Naive, memory, and effector CD8+ T cells express CD27highCD28+CD45RA+, CD27+CD28+CD45RA−, and CD27−CD28−CD45RA+/− phenotypes, respectively.10-12 Cytolytic effector molecules, perforin, and granzyme A/B are considered to be markers for effector CD8+ T cells9-11 because they are the actual functional molecules for killing target cells.13 CD27+CD28+CD45RA− memory CD8+ T cells express granzyme A and very low levels of granzyme B and perforin, and do not have immediate cytolytic activity without in vitro stimulation.11-14 Previous studies have demonstrated that ex vivo CD8+ T cells with the CD27lowCD28−CD45RA+/− or CD27−CD28−CD45RA+/− phenotype have the ability to kill target cells10,14 and that CD27lowCD28−CD45RA+/− late effector memory subsets express granzyme A, granzyme B, and perforin, with approximately half of them expressing perforin at a high level.11 In contrast, CD27−CD28−CD8+ T cells express high levels of granzyme A, granzyme B, and perforin11 and possess strong cytolytic activity.10,14 This finding indicates that these CD27−CD28−CD8+ T cells are terminally differentiated effector CD8+ T cells. In chronically HIV-infected individuals, HIV-1–specific CD8+ T cells are barely detected in the CD27−CD28− effector subset, but are found in the CD27+CD28− subset, suggesting that this skewed differentiation of CD8+ T cells may be one of the reasons that HIV-1–specific cytotoxic T-lymphocytes (CTLs) cannot completely eradicate HIV-1 from the body.15,16 Therefore, the detailed functional analysis of human effector CD8+ T cells and their differentiation pathway is informative and helpful for the understanding of HIV-1 pathogenesis.
Chemokine receptors play an important role in lymphocyte trafficking and are also used to define the functional subsets of human CD8+ T cells. Naive and central memory CD8+ T cells express CCR7 for homing to secondary lymph nodes,17 whereas effector memory and effector CD8+ T cells express the chemokine receptors for inflammatory cytokines, which enable the cells to migrate toward infected and inflamed sites. A unique subset of the effector CD8+ T-cell population expresses CXCR1.18,19 These CXCR1+CD8+ T cells possess chemotactic activity toward the CXCR1 ligand IL-8,18,19 a potent inflammatory cytokine produced in inflamed tissues and in tissues infected with some viruses, such as human cytomegalovirus (HCMV) or influenza A.20,21 This evidence suggests that these CXCR1+ effector CD8+ T cells immediately migrate to inflamed and infected sites to exert their effector function in the initial stage of an immune response. These data raise the possibility that effector CD8+ T-cell subsets are functionally distinct populations.
In the present study, we focused on the functional heterogeneity of human effector CD8+ T-cell subsets having actual effector functions. For this purpose, we first classified effector CD27−CD28−CD8+ T cells based on their CXCR1 expression and then analyzed the functional differences between these subsets. We further analyzed the gene-expression profile of these 2 functionally distinct effector CD8+ T-cell subsets. Our results reveal a novel subset of human effector CD8+ T cells that can produce IL-2.
Methods
Blood samples
All blood samples were taken from healthy, adult subjects with informed consent in accordance with the Declaration of Helsinki using a procedure based on the protocol approved by the ethics committee of Kumamoto University. PBMCs were isolated from peripheral blood using Ficoll-Paque PLUS (GE Healthcare Life Sciences).
Cells
C1R cells expressing HLA-A*0201 and HLA-A*0206 (C1R-A*0201 and C1R-A*0206) were generated as described previously22 and maintained in RPMI-1640 medium supplemented with 5% FCS and 0.15 mg/mL of hygromycin B.
HLA-class I tetramers
Flow cytometric analysis
All cell surface, intracellular, and tetramer staining procedures were performed as described previously.11,18 Information about each Ab is available in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To measure cytokine production in CXCR1+ or CXCR1− CD27−CD28−CD8+ T cells, we purified these subsets by cell sorting. The sorted T-cell subsets were cultured for 6 hours in flat-bottom 96-well plates with or without phorbol 12-myristate 13-acetate (PMA; 10 ng/mL)/ ionomycin (1 μg/mL) in RPMI-1640 medium supplemented with 10% FCS (R10 medium). After the first 2 hours of incubation, brefeldin A (10 μg/mL) was added to each well. To measure cytokine production in HCMV-specific CD8+ T cells, we cocultured the sorted T-cell subsets with C1R-A*0201 or C1R-A*0206 cells pulsed with or without HCMV-1 CTL epitope peptide for 6 hours. For the CaMKIV inhibition assay, PBMCs were pretreated for 30 minutes with STO-609 (Sigma-Aldrich) dissolved in DMSO, and then stimulated for 4 hours in anti-CD3 mAb–coated plates. For the condition without inhibitor, the same amount of DMSO was added. To detect phosphorylated STAT5, we stimulated FACS-sorted cells for 15 minutes at 37°C in the presence of recombinant human IL-7 (rhIL-7; 10 ng/mL, PeproTech) in R10 medium. After stimulation, the cells were fixed for 10 minutes at 37°C using 4% paraformaldehyde PBS, pelleted, and made permeable in PERM buffer III (BD Biosciences) for 30 minutes on ice. After washing, the cells were stained with anti-phosphorylated STAT5 (anti-pSTAT5) mAb for 30 minutes at room temperature. The stained cells were analyzed by flow cytometry (FACSAria or FACSCanto II; BD Biosciences). All flow cytometric data were analyzed using FlowJo Version 8 software (TreeStar).
FACS
To purify the CXCR1+ and CXCR1− effector CD8+ cells, we first isolated CD8+ T cells from PBMCs using anti-CD8–coated (clone: BW135/80) magnetic beads (Miltenyi Biotec). The isolated CD8+ T cells (> 98%) were further purified by staining with anti-CD8 mAb (clone: RPA-T8) for FACS sorting. CXCR1+CD27−CD28−CD8high and CXCR1−CD27−CD28−CD8high cells were sorted using the FACSAria flow cytometer.
CD8+ T-cell proliferation assay
CFSE (Molecular Probes) labeling was performed as described previously.11 CFSE-labeled sorted CD8+ T-cell subsets were cultured for 5 days on anti-CD3 (5 μg/mL) mAb-coated flat-bottomed 96-well plates in R10 medium supplemented or not with 200 U/mL of rhIL-2. For HCMV-specific CD8+ T cells, CFSE-labeled sorted cells were cocultured for 5 days with irradiated (150 Gy) C1R-A*0201 cells pulsed with or without HCMV-1 CTL epitope peptide in the medium supplemented or not with 200 U/mL of rhIL-2. To exclude the dead cells, we added 7-amino-actinomycin D (BD Biosciences) after the cell-surface staining.
Assay for cytotoxic activity
The cytotoxic activity of HCMV-specific CTLs was measured by the standard 51Cr-release assay, as described previously.11 FACS-sorted CD8+ T cells were used as effector cells. Effector/target ratios were determined by the percentage of HCMV-1-A*0201 tetramer–positive cells in each subset of CD8+ T cells.
RNA amplification and array processing
Total RNA was extracted from FACS-sorted CXCR1+ and CXCR1− subsets from 5 healthy individuals using an RNeasy Plus Micro Kit (QIAGEN). We confirmed the integrity and concentration of the total RNA by use of an RNA 6000 Pico kit and a Lab-on-a-Chip 2100 Bioanalyzer (Agilent Technologies). We amplified 16-50 ng of total RNA (RNA Integrity Number > 8.5) as single-stranded cDNA using a WT-Ovation Pico RNA Amplification System (NuGEN). Amplified cDNA yields were 6.3-10.1 μg with A260:A280 ratios of > 1.9. The amplified cDNA (5 μg) was fragmented and then labeled with biotin using the FL-Ovation cDNA Biotin Module V2 (NuGEN). Biotin-labeled cDNA was hybridized to Affymetrix HG-U133 Plus 2.0 arrays. The arrays were washed and then scanned with an Affymetrix 7G system. Scanning and initial analysis were performed using Affymetrix GeneChip Version 1.4 operating software. The percentage of genes determined by the GeneChip software to be present on the array was 49.5%-53.0%. GAPDH 3′ to 5′ ratios ranged from 0.80 to 1.51.
Microarray data analysis
GeneChip data analysis was performed using GeneSpring GX Version 9.0 software (Agilent Technologies) as follows: scanned CEL files were imported into GeneSpring software and then normalized with the robust multichip averaging. The probes showing the lowest 20% of signals among the total probes were excluded from further analysis; the remaining probes were subjected to unpaired Mann-Whitney test with a 1000 times permutation test to reduce the false discovery. The probes that detected more than 2-fold higher levels relative to each subset with statistical significance (P < .01) were listed. The microarray data have been deposited in the Gene Expression Omnibus Web site and can be accessed at http://www.ncbi.nlm.nih.gov/projects/geo/ (query GSE 26890).
Quantitative real-time PCR
Total RNA was extracted from FACS-sorted naive, memory, CXCR1+, and CXCR1− subsets from 4 healthy subjects using an RNeasy Plus Micro kit (QIAGEN). RNA was reverse-transcribed with random primers (High-Capacity cDNA Reverse Transcription kit; Applied Biosystems). Presynthesized TaqMan gene-expression assays were used to amplify all target genes using a 7500 Real-Time PCR System (Applied Biosystems), and each assay ID is available in supplemental Methods. Target gene values were expressed relative to the value for hypoxanthine phosphoribosyltransferase, with the values of the subset showing the highest expression of each gene standardized at 100% in each individual.
Statistics analysis
Each statistical analysis was performed by the 2-tailed paired (for the IL-2 effect and the IL-7 treatment) or unpaired unequal variances Student t test. A probability value of P < .05 was considered statistically significant.
Results
Both CXCR1+ and CXCR1− subsets of CD27−CD28−CD8+ T cells are functional cytolytic effector CD8+ T cells
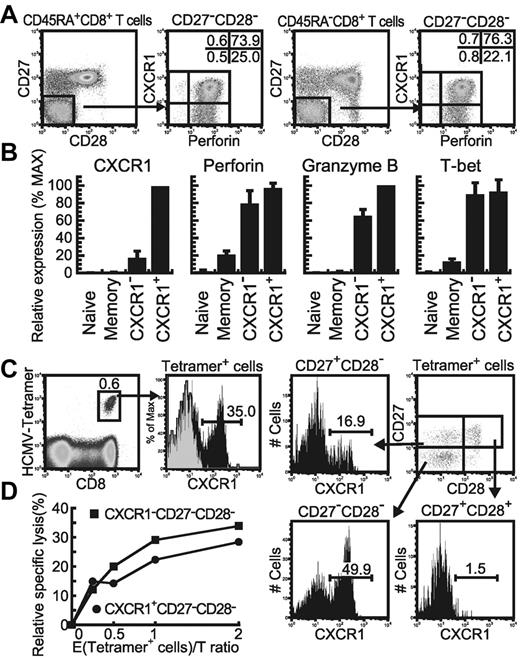
Both CXCR1+ and CXCR1− subsets of CD27−CD28−CD45RA+/− effector CD8+ T cells expressed a high level of perforin (Figure 1A), supporting previous studies showing that the chemokine receptor CXCR1 is exclusively expressed on effector CD27−CD28−CD45RA+/− CD8+ T cells having potent cytolytic activity.18,19 To investigate whether these 2 subsets have any differences in effector functions, we first analyzed the expression profiles of the Tbx21 gene, which encodes a key transcriptional factor, T box expressed in T cells (T-bet), which allows effector CD8+ T cells to induce the expression of IFN-γ, perforin, and granzyme B.25,26 The naive subset did not express T-bet, and the memory subset only slightly expressed it. In contrast, both the CXCR1+ and CXCR1− effector subsets expressed T-bet at a high level and also expressed perforin and granzyme B equivalently (Figure 1B), suggesting no difference in the expression of these effector CD8+ T-cell–associated genes between these 2 subsets. Consistent with previous studies,18 CXCR1+ cells were predominantly found in the CD27−CD28− subset of HCMV-specific CD8+ T cells (Figure 1C). When we compared the cytotoxic activity against target cells pulsed with HCMV epitope peptide between them, the CXCR1+ and CXCR1− subsets had similar potent cytolytic activity (Figure 1D), indicating that both subsets are functional effector CD8+ T cells.
CXCR1 expression on effector subsets in human peripheral CD8+ T cells. (A) Differential expression of perforin and CXCR1 on/in CD27−CD28−CD45RA+/− subsets of human CD8+ T cells in freshly isolated PBMCs is shown as 1 representative result from 4 healthy individuals. The values in each plot show the frequency of each population. (B) Gene-expression profiles of CXCR1+ and CXCR1− cells in the CD27−CD28− effector subset, the CD27highCD28+CD45RA+ naive subset, and the CD27+CD28+CD45RA− memory subset are shown as the mean relative gene-expression levels and SD from 4 healthy individuals. (C) HCMV-1-A*0201 tetramer+ cells among PBMCs from a HCMV-seropositive healthy individual (U-14) were analyzed for their CXCR1 expression. The percentage of CXCR1+ cells in each CD27CD28 subset of tetramer-positive cells was also determined. (D) Cytolytic activities of CXCR1+ and CXCR1− cells in the effector subset toward C1R-A*0201 cells prepulsed with the HCMV-1 pp65 495-503 peptide.
CXCR1 expression on effector subsets in human peripheral CD8+ T cells. (A) Differential expression of perforin and CXCR1 on/in CD27−CD28−CD45RA+/− subsets of human CD8+ T cells in freshly isolated PBMCs is shown as 1 representative result from 4 healthy individuals. The values in each plot show the frequency of each population. (B) Gene-expression profiles of CXCR1+ and CXCR1− cells in the CD27−CD28− effector subset, the CD27highCD28+CD45RA+ naive subset, and the CD27+CD28+CD45RA− memory subset are shown as the mean relative gene-expression levels and SD from 4 healthy individuals. (C) HCMV-1-A*0201 tetramer+ cells among PBMCs from a HCMV-seropositive healthy individual (U-14) were analyzed for their CXCR1 expression. The percentage of CXCR1+ cells in each CD27CD28 subset of tetramer-positive cells was also determined. (D) Cytolytic activities of CXCR1+ and CXCR1− cells in the effector subset toward C1R-A*0201 cells prepulsed with the HCMV-1 pp65 495-503 peptide.
The CXCR1−CD27−CD28− effector CD8+ T-cell subset retains its potential to secrete IL-2
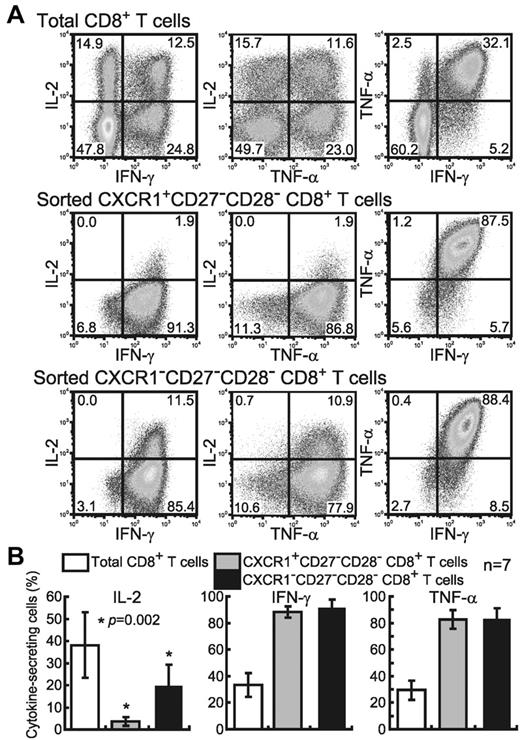
Effector cytokines such as IFN-γ and TNF-α are predominantly produced by memory effector and effector CD8+ T cells, whereas IL-2 is predominantly secreted by memory CD8+ T cells,9,14,27 indicating that the differences in the production of these cytokines reflect the maturation and differentiation states of CD8+ T cells. To clarify the functional differences in cytokine production between the CXCR1+ and CXCR1− effector T cells, we investigated the ability of these cells to produce IL-2, IFN-γ, and TNF-α. Because the surface expression of CXCR1 on CD8+ T cells is down-regulated according to activation via the TCR,19 we stimulated isolated CXCR1+ and CXCR1− effector subsets with PMA and ionomycin (Figure 2A-B). Both CXCR1+ and CXCR1− effector subsets produced a large amount of the effector cytokines IFN-γ (88.3% ± 4.0% and 91.7% ± 5.9%, respectively) and TNF-α (82.6% ± 6.9% and 83.0% ± 7.8%, respectively). In contrast, the CXCR1+ subset secreted significantly less IL-2 (3.7% ± 2.1%, P = .002) than the CXCR1− subset (19.8% ± 9.5%). These IL-2–producing CXCR1− cells secreted both IFN-γ and TNF-α at high levels, suggesting that CXCR1− cells have effector function.
Different expression of 3 cytokines in CXCR1+ and CXCR1− effector subsets of CD8+ T cells. (A) FACS-sorted CXCR1+ and CXCR1− CD27−CD28− CD8+ T cells from healthy subject number U-14 were stimulated for 6 hours with PMA and ionomycin to determine the percentage of each kind of cytokine-producing cell. (B) The mean percentage and SD of each type of PMA and ionomycin-induced cytokine-producing cell in each CD8+ T-cell subset from 7 individuals are shown.
Different expression of 3 cytokines in CXCR1+ and CXCR1− effector subsets of CD8+ T cells. (A) FACS-sorted CXCR1+ and CXCR1− CD27−CD28− CD8+ T cells from healthy subject number U-14 were stimulated for 6 hours with PMA and ionomycin to determine the percentage of each kind of cytokine-producing cell. (B) The mean percentage and SD of each type of PMA and ionomycin-induced cytokine-producing cell in each CD8+ T-cell subset from 7 individuals are shown.
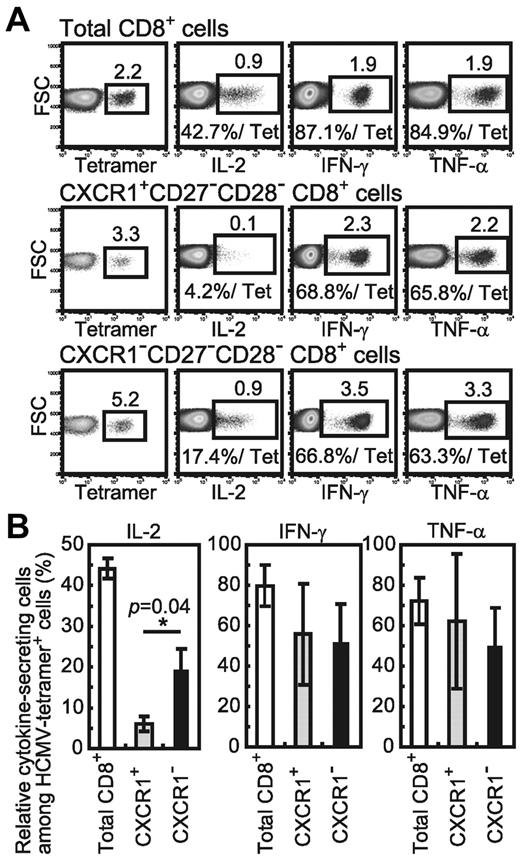
To clarify the differences in cytokine production between these 2 subsets of virus-specific CD8+ T cells, we analyzed the ability of the subsets among HCMV-specific CD8+ T cells to produce cytokines (Figure 3A-B). The CXCR1+ subset of HCMV-specific CD8+ T cells failed to produce IL-2 (6.1% ± 1.8%), whereas the CXCR1− cells did do so (19.2% ± 5.2%). In contrast, a substantial percentage of both subsets of HCMV-specific CD8+ T cells produced IFN-γ and TNF-α. Therefore, HCMV-specific CXCR1−CD27−CD28−CD8+ T cells had the ability to produce IL-2, whereas the HCMV-specific CXCR1+CD27−CD28−CD8+ T cells had lost it.
Different cytokine productivity of CXCR1+ and CXCR1− effector subsets among HCMV-specific CD8+ T cells. (A) Cytokine productivity of HCMV-specific effector CD8+ T cells. CXCR1+ and CXCR1− cells in the CD27−CD28−CD8+ cell population were sorted from HCMV-seropositive healthy subject number U-14. Sorted cells were cocultured with C1R-A*0201 cells pulsed with HCMV-1 CTL epitope peptide for 6 hours, and then the frequency of cytokine-positive cells was determined by staining for intracellular cytokine. These cells were also stained with HCMV-tetramer in tubes separate from those used for the cytokine staining to detect the frequency of HCMV-specific CD8+ cells. The frequency of cytokine-producing cells in the total HCMV-specific cell population of each subset is shown as the percentage of cytokine-positive cells relative to the percentage of the HCMV-tetramer+ cell population (%/Tet). Nonspecific cytokine-positive cells (ie, cytokine-positive cells in the coculture of C1R-A*0201 cells without peptide) were subtracted from all data. (B) The mean percentage and SD of cells producing each type of cytokine in each HCMV-specific CD8+ T-cell subset from 3 HCMV-seropositive healthy subjects are shown.
Different cytokine productivity of CXCR1+ and CXCR1− effector subsets among HCMV-specific CD8+ T cells. (A) Cytokine productivity of HCMV-specific effector CD8+ T cells. CXCR1+ and CXCR1− cells in the CD27−CD28−CD8+ cell population were sorted from HCMV-seropositive healthy subject number U-14. Sorted cells were cocultured with C1R-A*0201 cells pulsed with HCMV-1 CTL epitope peptide for 6 hours, and then the frequency of cytokine-positive cells was determined by staining for intracellular cytokine. These cells were also stained with HCMV-tetramer in tubes separate from those used for the cytokine staining to detect the frequency of HCMV-specific CD8+ cells. The frequency of cytokine-producing cells in the total HCMV-specific cell population of each subset is shown as the percentage of cytokine-positive cells relative to the percentage of the HCMV-tetramer+ cell population (%/Tet). Nonspecific cytokine-positive cells (ie, cytokine-positive cells in the coculture of C1R-A*0201 cells without peptide) were subtracted from all data. (B) The mean percentage and SD of cells producing each type of cytokine in each HCMV-specific CD8+ T-cell subset from 3 HCMV-seropositive healthy subjects are shown.
Proliferation potency of CXCR1+ and CXCR1− effector CD8+ T cells
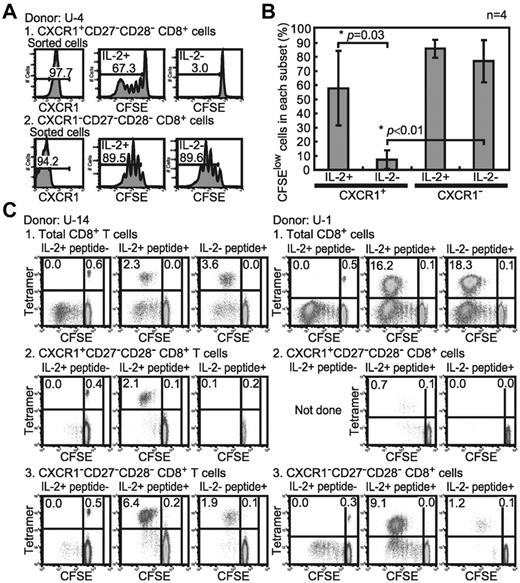
IL-2 is the most crucial growth factor for activated T cells to support their expansion and survival.28,29 We therefore speculated that CXCR1−CD27−CD28− cells would be able to self-propagate after Ag stimulation, whereas CXCR1+CD27−CD28− cells would not. We analyzed the proliferative capacity of both subsets responding to anti-CD3 mAb stimulation with or without exogenous IL-2 (Figure 4A-B). Both CXCR1+ and CXCR1− subsets proliferated when they were cultured in medium containing IL-2 (CFSElow cells: 57.8% ± 26.5% and 85.8% ± 6.4%, respectively). In contrast, when cultured without IL-2, the CXCR1− subset proliferated significantly (CFSElow cells: 76.9% ± 15.1%, P = .001), whereas the CXCR1+ subset failed to do so (CFSElow cells: 7.2% ± 6.5%). These results indicate that activated CXCR1−CD27−CD28−CD8+ T cells can proliferate regardless of exogenous IL-2, whereas activated CXCR1+CD27−CD28−CD8+ T cells require exogenous cytokines to proliferate.
Proliferation potency of CXCR1+ and CXCR1− effector CD8+ T cells. (A) TCR engagement–induced proliferation of effector CD8+ T cells. The CXCR1+ and CXCR1− subsets were sorted from healthy subject no. U-4. The CFSE-labeled sorted CD8+ T-cell subsets were analyzed for CFSE dilution profiles after 5 days of solid-phase anti-CD3 mAb stimulation with or without IL-2 (200 u/mL). The values in each histogram show the percentage of divided cells (CFSElow) and undivided cells (CFSEhigh). (B) Mean percentage and SD of CFSElow and CFSEhigh cells in each CD8+ T-cell subset after 5 days of anti-CD3 mAb stimulation from 4 individuals are shown. (C) Ag-driven proliferation of CXCR1+ and CXCR1− effector subsets. The effector CXCR1+ and CXCR1−subsets were sorted from the HCMV-seropositive healthy subjects U-14 and U-1. The percentage of HCVM-tetramer+ CD8+ T cells in each subset before stimulation is as follows: U-14 total CD8+ T cells: 2.2%, CXCR1+ subset: 3.3%, CXCR1− subset: 5.2%; U-1 total CD8+ T cells: 2.4%, CXCR1+ subset: 0.5%, and CXCR1− subset: 1.3%. The CFSE-labeled sorted CD8+ T-cell subsets were cocultured for 5 days with irradiated C1R-A*0201 cells pulsed or not with HCMV-1 CTL epitope peptide. The percentages of divided HCMV-1-A*0201 tetramer+ (top left), divided tetramer− (bottom left), undivided tetramer+ (top right), and undivided tetramer− (bottom right) cells in each sorted subset are shown.
Proliferation potency of CXCR1+ and CXCR1− effector CD8+ T cells. (A) TCR engagement–induced proliferation of effector CD8+ T cells. The CXCR1+ and CXCR1− subsets were sorted from healthy subject no. U-4. The CFSE-labeled sorted CD8+ T-cell subsets were analyzed for CFSE dilution profiles after 5 days of solid-phase anti-CD3 mAb stimulation with or without IL-2 (200 u/mL). The values in each histogram show the percentage of divided cells (CFSElow) and undivided cells (CFSEhigh). (B) Mean percentage and SD of CFSElow and CFSEhigh cells in each CD8+ T-cell subset after 5 days of anti-CD3 mAb stimulation from 4 individuals are shown. (C) Ag-driven proliferation of CXCR1+ and CXCR1− effector subsets. The effector CXCR1+ and CXCR1−subsets were sorted from the HCMV-seropositive healthy subjects U-14 and U-1. The percentage of HCVM-tetramer+ CD8+ T cells in each subset before stimulation is as follows: U-14 total CD8+ T cells: 2.2%, CXCR1+ subset: 3.3%, CXCR1− subset: 5.2%; U-1 total CD8+ T cells: 2.4%, CXCR1+ subset: 0.5%, and CXCR1− subset: 1.3%. The CFSE-labeled sorted CD8+ T-cell subsets were cocultured for 5 days with irradiated C1R-A*0201 cells pulsed or not with HCMV-1 CTL epitope peptide. The percentages of divided HCMV-1-A*0201 tetramer+ (top left), divided tetramer− (bottom left), undivided tetramer+ (top right), and undivided tetramer− (bottom right) cells in each sorted subset are shown.
We also analyzed the expression of IL-2 receptors on these effector subsets to determine whether their IL-2 reactivity was because of a difference in IL-2 receptor expression (supplemental Figure 1A-B). Both CXCR1+ and CXCR1− effector subsets expressed CD122 (IL-2R β chain) at a similar level, although some of the cells in the naive and memory subsets did also. Conversely, neither effector subset expressed activation marker CD25 (IL-2R α chain). However, the CXCR1+ and CXCR1− subsets up-regulated CD25 expression similarly after stimulation with anti-CD3 (supplemental Figure 1C). These results suggest that CXCR1+ and CXCR1− effector CD8+ T-cell subsets possess a similar IL-2 reactivity, and that the IL-2 productivity of each subset reflects the self-proliferative capacity of the subsets.
We also investigated the correlation between the expression of CXCR1 and PD-1 on CD8+ T cells, because PD-1 expression affects functions such as IL-2 productivity and proliferative potency.30,31 However, the CXCR1− subset contained more cells expressing PD-1 than the CXCR1+ subset (supplemental Figure 2), indicating that the loss of IL-2 productivity was not because of the expression of PD-1.
To clarify whether the difference in IL-2 production affected the Ag-driven proliferation of virus-specific effector CD8+ T cells, we analyzed the proliferative capacity of CXCR1+ and CXCR1− HCMV-specific effector CD8+ T cells. Both the CXCR1+ and CXCR1− subsets of HCMV-specific effector CD8+ T cells effectively proliferated after Ag stimulation in the presence of IL-2 (Figure 4C). After Ag stimulation in the absence of IL-2, the CXCR1+ subset failed to proliferate, whereas the CXCR1− subset did proliferate. These results indicate that the CXCR1− subset of effector CD8+ T cells can proliferate autonomously after Ag stimulation in the absence of an exogenous cytokine such as IL-2, in contrast to the CXCR1+ cells. Although it is not clear that only IL-2 is required for the proliferation of these effector CD8+ T cells, our data suggest that IL-2 productivity of the CXCR1− effector cells plays an important role in the self-proliferation of virus-specific effector CD8+ T cells.
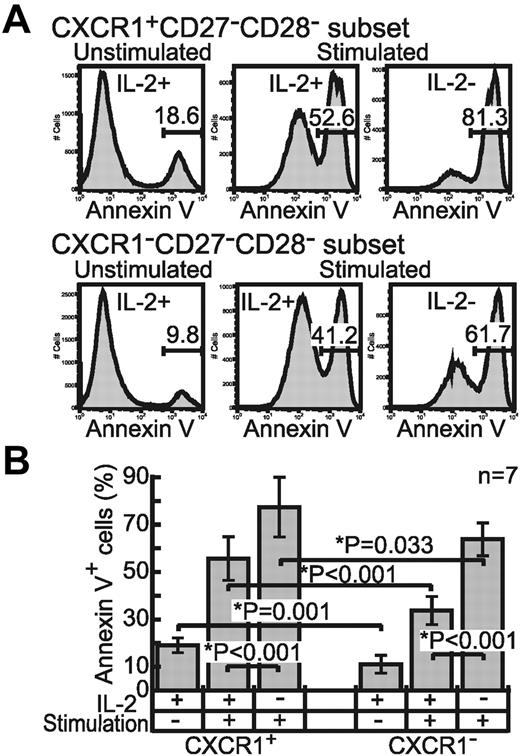
Susceptibility of effector CD8+ T-cell subsets to dying
Judging from the IL-2 productivity and proliferative capacity of the 2 subsets, we assumed that the CXCR1+ subset, not the CXCR1− subset, represented terminally differentiated effector CD8+ T cells. Terminally differentiated CD8+ T cells are thought to be more susceptible to activation-induced cell death (AICD).32,33 Therefore, we investigated the susceptibility of these 2 effector subsets to AICD after stimulating them on anti-CD3 mAb-coated plates in the presence or absence of IL-2. After the stimulation, there were distinct peaks of the cells that showed annexin V binding at a high level (Figure 5A). We regarded the annexin V high-binding cells (annexin V+) as dying cells, because such cells were reported as dead cells in the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, whereas the annexin V low-binding cells were not.34 The subsets not stimulated with exogenous IL-2 contained more annexin V+ cells than those incubated with IL-2 (CXCR1+ subset: P < .001, CXCR1− subset: P < .001), indicating that exogenous IL-2 reduced the number of annexin V+ cells in both subsets. Under all culture conditions, the percentage of annexin V+ cells was significantly higher in the CXCR1+ subset than in the CXCR1− subset (Figure 5B). These findings indicate that IL-2 supports the survival of effector CD8+ T cells and that the CXCR1− cells are more resistant to AICD than CXCR1+ cells.
Difference between effector CD8+ T-cell subsets in terms of susceptibility to dying. (A) Purified CXCR1+ and CXCR1− effector subsets were stimulated or not with solid-phase anti-CD3 mAb and cultured with or without IL-2 (200 U/mL) for 48 hours. Doublet cells were excluded from the CD3+CD8+ population and then the percentage of annexin V+ cells was analyzed. (B) The mean percentage of annexin V+ cells in each effector subset from 7 individuals is shown with the SD. *Significant statistical difference at the indicated level.
Difference between effector CD8+ T-cell subsets in terms of susceptibility to dying. (A) Purified CXCR1+ and CXCR1− effector subsets were stimulated or not with solid-phase anti-CD3 mAb and cultured with or without IL-2 (200 U/mL) for 48 hours. Doublet cells were excluded from the CD3+CD8+ population and then the percentage of annexin V+ cells was analyzed. (B) The mean percentage of annexin V+ cells in each effector subset from 7 individuals is shown with the SD. *Significant statistical difference at the indicated level.
Gene-expression profiles of effector CD8+ T-cell subsets
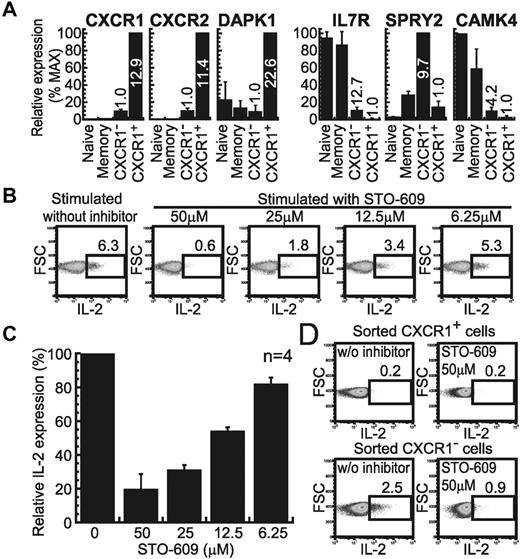
We performed microarray gene-expression analysis to find the genes that could be responsible for the difference in IL-2 productivity and cell survival between the CXCR1+ and CXCR1− subsets. We highly purified each CD3+CD8+ subset for this analysis (> 95.3%), and then compared the gene-expression profile of each subset. Of the probes used, 43 891 (99.8%) detected genes with a difference in expression of less than 2-fold relative to each subset or did not show a statistically higher level; average-linkage hierarchical clustering categorized these samples into each donor but not into each subset (data not shown), suggesting that these 2 subsets were functionally very close. However, we found 21 probes that detected at least a 2-fold higher level relative to each subset, with statistical significance (P < .01) in all 5 subjects examined: 7 probes (6 genes) for up-regulated gene expression in the CXCR1+ subset (Table 1) and 14 probes (12 genes) for the CXCR1− subset (Table 2). Sixty-five probes also satisfied the same criteria when the average of 5 healthy individuals was examined (supplemental Tables 1 and 2). First, a difference in the expression of chemokine receptor–associated genes was found between these subsets: the CXCR1+ subset showed up-regulated expression of IL8RA (CXCR1) and the other low-affinity IL-8 receptor IL8RB (CXCR2), and G Protein GNAL (Table 1), whereas the CXCR1− subset revealed up-regulated expression of the memory T-cell–expressing CXCR6 and CCR5 (supplemental Table 2). Second, the pro-apoptotic death-associated protein kinase 1 (DAPK1) was up-regulated in the CXCR1+ subset (supplemental Table 1), whereas prosurvival SPRY2 and IL-7R were up-regulated in the CXCR1− subset (Table 2). Finally, the CXCR1− subset expressed the IL-2–inducible calmodulin-dependent protein kinase IV (CAMK4 or CaMKIV; Table 2). Quantitative PCR revealed that the CXCR1+ subset expressed DAPK1 at a clearly higher level than the CXCR1− subset (Figure 6A), as opposed to its low -fold change seen in the GeneChip data. SPRY2 expression was exclusively found in the CXCR1− subset (Figure 6A). The up-regulation of IL-7R and CAMK4 in the CXCR1− subset was also confirmed by this assay (Figure 6A; 12.7- and 4.2-fold relative to the CXCR1+ subset with statistical significance P = .01 and P = .02, respectively), although their highest expression was found in the naive and memory subsets. These results indicate that the CXCR1+ subset expresses pro-apoptotic genes and that the CXCR1− subset expresses prosurvival and IL-2–inducible genes.
Genes up-regulated in the CXCR1+ subset compared with those in the CXCR1− subset
| Fold change . | Gene symbol . | Gene name . | Description . |
|---|---|---|---|
| 5.50 | IL-8RB | Interleukin 8 receptor, beta | G-protein–coupled receptor |
| 5.06 | MPPE1 | Metallophosphoesterase 1 | Metallophosphoesterase |
| 4.62 | IL-8RA | Interleukin 8 receptor, alpha | G-protein–coupled receptor |
| 4.39 | MPPE1 | Metallophosphoesterase 1 | Metallophosphoesterase |
| 4.12 | GNAL | Guanine nucleotide binding protein (G protein), alpha activating activity polypeptide, olfactory type | Guanine nucleotide binding protein |
| 3.86 | DNAH10 | Dynein, axonemal, heavy chain 10 | Microtubule-associated motor protein |
| 3.19 | FRMD4B | FERM domain containing 4B | Cytoskeletal-associated protein |
| Fold change . | Gene symbol . | Gene name . | Description . |
|---|---|---|---|
| 5.50 | IL-8RB | Interleukin 8 receptor, beta | G-protein–coupled receptor |
| 5.06 | MPPE1 | Metallophosphoesterase 1 | Metallophosphoesterase |
| 4.62 | IL-8RA | Interleukin 8 receptor, alpha | G-protein–coupled receptor |
| 4.39 | MPPE1 | Metallophosphoesterase 1 | Metallophosphoesterase |
| 4.12 | GNAL | Guanine nucleotide binding protein (G protein), alpha activating activity polypeptide, olfactory type | Guanine nucleotide binding protein |
| 3.86 | DNAH10 | Dynein, axonemal, heavy chain 10 | Microtubule-associated motor protein |
| 3.19 | FRMD4B | FERM domain containing 4B | Cytoskeletal-associated protein |
Genes up-regulated more than 2-fold in all individuals, with statistical significance at P < .01.
Genes up-regulated in CXCR1− subset compared with those in the CXCR1+ subset
| Fold change . | Gene symbol . | Gene name . | Description . |
|---|---|---|---|
| 17.75 | GZMK | Granzyme K (granzyme 3; tryptase II) | Cytolytic serine protease |
| 6.88 | IL-7R | Interleukin 7 receptor | Interleukin 7 receptor α chain |
| 6.69 | IL-7R | Interleukin 7 receptor | Interleukin 7 receptor α chain |
| 5.84 | VCAM1 | Vascular cell–adhesion molecule 1 | Cell-surface sialoglycoprotein (cell-adhesion molecule) |
| 4.76 | NELL2 | NEL-like 2 (chicken) | Cytoplasmic glycoprotein containing several von Willebrand factor C domains and epidermal growth factor (EGF)–like domains |
| 4.70 | RCAN3 | RCAN family member 3 | Regulator of calcineurin protein |
| 4.56 | SPRY1 | Sprouty homolog 1 (Drosophila) | Regulator of EGFR signaling |
| 4.48 | SPRY2 | Sprouty homolog 2 (Drosophila) | Regulator of EGFR signaling |
| 4.27 | CDCA7 | Cell division cycle–associated 7 | c-Myc–responsive transcription regulator |
| 3.83 | GPR183 | G protein–coupled receptor 183 | G protein–coupled receptor |
| 3.20 | DUSP4 | Dual specificity phosphatase 4 | Dual specificity protein phosphatase subfamily |
| 2.96 | RCAN3 | RCAN family member 3 | Regulators of calcineurin protein |
| 2.71 | TBC1D4 | TBC1 domain family, member 4 | Regulator of Rab GTPase activity |
| 2.62 | CAMK4 | Calcium/calmodulin-dependent protein kinase IV | Ca2+/calmodulin-dependent protein kinase |
| Fold change . | Gene symbol . | Gene name . | Description . |
|---|---|---|---|
| 17.75 | GZMK | Granzyme K (granzyme 3; tryptase II) | Cytolytic serine protease |
| 6.88 | IL-7R | Interleukin 7 receptor | Interleukin 7 receptor α chain |
| 6.69 | IL-7R | Interleukin 7 receptor | Interleukin 7 receptor α chain |
| 5.84 | VCAM1 | Vascular cell–adhesion molecule 1 | Cell-surface sialoglycoprotein (cell-adhesion molecule) |
| 4.76 | NELL2 | NEL-like 2 (chicken) | Cytoplasmic glycoprotein containing several von Willebrand factor C domains and epidermal growth factor (EGF)–like domains |
| 4.70 | RCAN3 | RCAN family member 3 | Regulator of calcineurin protein |
| 4.56 | SPRY1 | Sprouty homolog 1 (Drosophila) | Regulator of EGFR signaling |
| 4.48 | SPRY2 | Sprouty homolog 2 (Drosophila) | Regulator of EGFR signaling |
| 4.27 | CDCA7 | Cell division cycle–associated 7 | c-Myc–responsive transcription regulator |
| 3.83 | GPR183 | G protein–coupled receptor 183 | G protein–coupled receptor |
| 3.20 | DUSP4 | Dual specificity phosphatase 4 | Dual specificity protein phosphatase subfamily |
| 2.96 | RCAN3 | RCAN family member 3 | Regulators of calcineurin protein |
| 2.71 | TBC1D4 | TBC1 domain family, member 4 | Regulator of Rab GTPase activity |
| 2.62 | CAMK4 | Calcium/calmodulin-dependent protein kinase IV | Ca2+/calmodulin-dependent protein kinase |
Genes up-regulated more than 2-fold in all individuals, with statistical significance at P < .01.
Different gene-expression profiles between effector CD8+ T-cell subsets. (A) Quantitative PCR for the indicated genes up-regulated in CXCR1+ (left) or CXCR1− (right) subsets was performed with FACS-sorted CXCR1+ and CXCR1− cells in the effector, naive, and memory subsets. The mean relative gene-expression levels and SD from 4 healthy individuals are shown. The values on each column show the relative gene-expression ratio between the 2 subsets; left: up-regulated genes in CXCR1+ cells, right: those in CXCR1− cells. (B) PBMCs from subject number U-43 were stimulated for 4 hours with solid-phase anti-CD3 mAb with or without STO-609. The percentage of IL-2–producing cells in the CD3+CD8+CD27−CD28− subset is shown. (C) The same experiment as in panel B was performed with the PBMCs from 4 healthy subjects, and then the relative percentage of IL-2–producing cells was expressed as the mean and SD based on that in the condition without inhibitor. (D) The CXCR1+ and CXCR1− cells in the CD27−CD28− effector CD8+ T-cell subset were sorted and then the cells were stimulated with solid-phase anti-CD3 mAb with or without STO-609 to assess the percentage of IL-2–producing cells.
Different gene-expression profiles between effector CD8+ T-cell subsets. (A) Quantitative PCR for the indicated genes up-regulated in CXCR1+ (left) or CXCR1− (right) subsets was performed with FACS-sorted CXCR1+ and CXCR1− cells in the effector, naive, and memory subsets. The mean relative gene-expression levels and SD from 4 healthy individuals are shown. The values on each column show the relative gene-expression ratio between the 2 subsets; left: up-regulated genes in CXCR1+ cells, right: those in CXCR1− cells. (B) PBMCs from subject number U-43 were stimulated for 4 hours with solid-phase anti-CD3 mAb with or without STO-609. The percentage of IL-2–producing cells in the CD3+CD8+CD27−CD28− subset is shown. (C) The same experiment as in panel B was performed with the PBMCs from 4 healthy subjects, and then the relative percentage of IL-2–producing cells was expressed as the mean and SD based on that in the condition without inhibitor. (D) The CXCR1+ and CXCR1− cells in the CD27−CD28− effector CD8+ T-cell subset were sorted and then the cells were stimulated with solid-phase anti-CD3 mAb with or without STO-609 to assess the percentage of IL-2–producing cells.
To confirm CaMKIV-induced IL-2 production in the CXCR1− subset, we investigated the effect of the CaMKIV-inhibitor STO-609 on the anti-CD3 mAb-induced IL-2 production from the effector CD27−CD28−CD8+ T-cell subset. The results showed that this inhibitor dose-dependently abolished the IL-2 production (Figure 6B-C). IL-2 production from the sorted CXCR1− subset was also inhibited by the CaMKIV inhibitor (Figure 6D), indicating that the CXCR1− cells produced IL-2 by activating CaMKIV through TCR signaling.
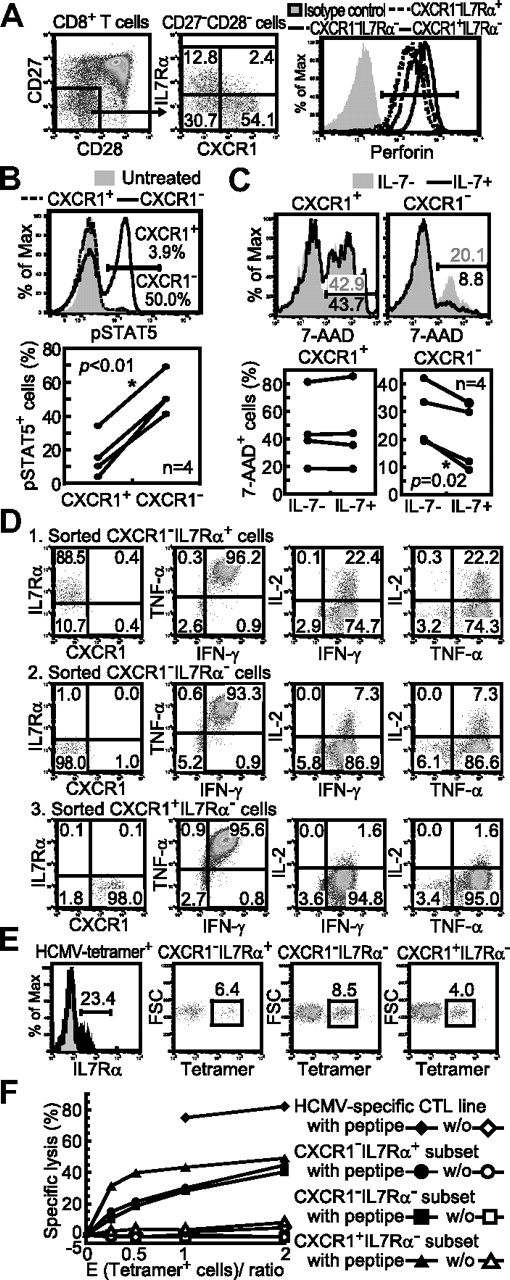
The CXCR1−IL-7Rα+ subset represents IL-2–producing effector CD8+ T cells
To confirm the expression of IL-7R on the CXCR1− subset of CD27−CD28− effector cells at the protein level, we examined the expression of IL-7Rα on the CXCR1− subset. Flow cytometric analysis demonstrated that IL-7Rα was expressed on approximately 30% of CXCR1− cells and that the CXCR1−IL-7Rα+ subset also expressed perforin at a high level (Figure 7A). We speculate that this IL-7R expression was important for the maintenance of the CXCR1− effector CD8+ T-cell subset, because the activation of STAT5 through JAK3 has been implicated in IL-7–dependent up-regulation of Bcl-2 to promote cell survival.35,36 IL-7 treatment significantly up-regulated the expression of pSTAT5 only in the CXCR1− subset (Figure 7B). Moreover, IL-7 promoted the survival of CXCR1− cells, but not that of CXCR1+ cells (Figure 7C). These results indicate that IL-7/IL-7R signaling prevents apoptosis of CXCR1− effector CD8+ T cells more effectively than that of CXCR1+ cells in vivo. We further assessed the cytokine productivity in this subset. Three effector subsets, CXCR1−IL-7Rα+, CXCR1−IL-7Rα−, and CXCR1+IL-7Rα−, equivalently produced the effector cytokines IFN-γ and TNF-α at a high level (Figure 7D). In contrast, only the CXCR1−IL-7Rα+ and the CXCR1−IL-7Rα− subsets produced IL-2, although at a much higher level in the former.
Functional property of IL-7R–expressing effector CD8+ T cells. (A) Perforin expression of CXCR1/IL-7Rα subsets in the CD27−CD28− effector CD8+ T-cell subset is shown as 1 representative result from 3 healthy individuals. (B) FACS-sorted CXCR1+ and CXCR1− effector subsets were treated with IL-7 (10 ng/mL) for 15 minutes, and then stained for pSTAT5 expression. Representative pSTAT5 staining with the percentage of pSTAT5+ cells in each subset and the percentage of pSTST5+ cells in each effector subset from 4 individuals are shown. (C) FACS-sorted CXCR1+ and CXCR1− effector subsets were cultured with or without IL-7 (5 ng/mL) for 48 hours, and then dead cells were detected by 7-amino-actinomycin D (7-AAD) staining. Representative results of 7-AAD staining with percentages of 7-AAD+ cells in each subset (without IL-7 in gray and with IL-7 in black) and the percentage of 7-AAD+ dead cells in each effector subset from 4 individuals shown. (D) FACS-sorted CXCR1/IL-7Rα subsets (far left plots) from healthy subject number U-14 were stimulated with PMA and ionomycin for 6 hours to assess the percentage of cytokine-producing cells. The flow plots are presented as a representative result from 3 healthy individuals showing similar results. (E) The CD8+, tetramer-positive cells from a HCMV-seropositive healthy subject (U-14) were analyzed for IL-7R expression. The frequency of tetramer-positive cells in each CXCR1/IL-7R effector CD8+ T-cell subset is shown. (F) CXCR1/IL-7Rα subsets were sorted to assess the cytolytic activity toward C1R-A*0201 cells prepulsed or not with the HCMV-1 pp65495-503 peptide.
Functional property of IL-7R–expressing effector CD8+ T cells. (A) Perforin expression of CXCR1/IL-7Rα subsets in the CD27−CD28− effector CD8+ T-cell subset is shown as 1 representative result from 3 healthy individuals. (B) FACS-sorted CXCR1+ and CXCR1− effector subsets were treated with IL-7 (10 ng/mL) for 15 minutes, and then stained for pSTAT5 expression. Representative pSTAT5 staining with the percentage of pSTAT5+ cells in each subset and the percentage of pSTST5+ cells in each effector subset from 4 individuals are shown. (C) FACS-sorted CXCR1+ and CXCR1− effector subsets were cultured with or without IL-7 (5 ng/mL) for 48 hours, and then dead cells were detected by 7-amino-actinomycin D (7-AAD) staining. Representative results of 7-AAD staining with percentages of 7-AAD+ cells in each subset (without IL-7 in gray and with IL-7 in black) and the percentage of 7-AAD+ dead cells in each effector subset from 4 individuals shown. (D) FACS-sorted CXCR1/IL-7Rα subsets (far left plots) from healthy subject number U-14 were stimulated with PMA and ionomycin for 6 hours to assess the percentage of cytokine-producing cells. The flow plots are presented as a representative result from 3 healthy individuals showing similar results. (E) The CD8+, tetramer-positive cells from a HCMV-seropositive healthy subject (U-14) were analyzed for IL-7R expression. The frequency of tetramer-positive cells in each CXCR1/IL-7R effector CD8+ T-cell subset is shown. (F) CXCR1/IL-7Rα subsets were sorted to assess the cytolytic activity toward C1R-A*0201 cells prepulsed or not with the HCMV-1 pp65495-503 peptide.
To clarify the ability of the CXCR1−L7Rα+ and CXCR1−IL-7Rα− subsets to kill target cells, we first sought to detect these subsets among HCMV-specific CD8+ T cells. Flow cytometric analysis using HLA-A*0201 tetramers demonstrated HCMV-specific T cells in these subsets (Figure 7E). Both the CXCR1−IL-7Rα+ and CXCR1−IL-7Rα− subsets showed similar, substantial HCMV-specific cytolytic ability comparable to that of the CXCR1+IL-7Rα− subset (Figure 7F), indicating that these 3 subsets were cytotoxic effector T cells. These results suggest that human effector CD8+ T cells consist of 3 functionally distinct subsets, CXCR1−IL-7Rα+ (IL-2 producer), CXCR1−IL-7Rα− (intermediate), and CXCR1+IL-7Rα− (the most differentiated) subsets.
Discussion
In the present study, we investigated 2 subsets of CD27−CD28− effector CD8+ T cells discriminated by CXCR1 expression on their surfaces. The CD27−CD28− subset was classified previously into highly differentiated effector CD8+ T cells based on the expression of perforin, granzyme A, and granzyme B at high levels and on cytotoxic function11 ; for this reason, both the CXCR1+ and CXCR1− subsets were thought to be effector CD8+ T cells. Indeed, both subsets expressed perforin and granzyme B at high levels and possessed immediate strong cytolytic activity ex vivo. Furthermore, our data showing the ability of these subsets to produce IFN-γ and TNF-α substantially support our contention that they are effector CD8+ T cells.
The CXCR1+ subset, which represents the majority of the CD27−CD28− effector CD8+ T-cell subset in healthy individuals without infections, lost their IL-2 productivity and their proliferation was dependent on exogenous IL-2. These data suggest that these cells are the most differentiated effector CD8+ T cells, and are likely maintained after an infection. Increased DAPK1 expression promotes apoptosis in response to various stimuli such as Fas, INF-γ, and TNF-α37,38 ; susceptibility to death is also thought to be a key feature of effector CD8+ T cells.32,33 We indeed demonstrated herein that the CXCR1+ subset was more susceptible to cell death than the CXCR1− subset in several conditions regardless of activation, and that DAPK1 was highly expressed in the CXCR1+ subset. These findings indicate that human CD8+ T cells with the CXCR1+CD27−CD28− phenotype are the most differentiated effector CD8+ T cells.
Conversely, the CXCR1− subset retained IL-2 productivity and had the ability to proliferate without exogenous IL-2. The CXCR1− subset was more resistant to dying than the CXCR1+ subset, even in cultures without activation, which is also consistent with the up-regulation of SPRY2 in the CXCR1− subset. SPRY2 is known as an essential modulator for serum-induced cell survival.39 Therefore, the results of the present study suggest that the CXCR1− subset are novel effector T cells possessing IL-2 productivity, self-proliferative ability, and survival capacity.
Effector CD8+ T cells highly express T-bet for their effector functions,25,26 whereas T-bet expression represses IL-2 productivity.40,41 In the present study, we showed that both CXCR1+ and CXCR1− subsets similarly expressed T-bet at a high level. This finding suggests that the difference in IL-2 productivity between CXCR1+ and CXCR1− subsets was not because of a difference in T-bet expression. Conversely, gene-expression analysis revealed that the CXCR1− subset expressed more CAMK4 than the CXCR1+ subset. In response to TCR-mediated signaling, CaMKIV is activated by CaM-kinase kinase, and the activated CaMKIV is then translocated to the nucleus to stimulate IL-2 transcription.42 STO-609 is known to be a CaM-kinase kinase inhibitor43 and actually repressed IL-2 production by the effector CD8+ T-cell subset. This result is consistent with a previous study showing that the IL-2 productivity of human primary CD4+ T cells is repressed by CAMK4 siRNA.44 These findings suggest that the expression of CaMKIV determines the IL-2 productivity in the CXCR1− effector CD8+ T cells.
We found that the CXCR1− cells exhibited up-regulation of genes that are expressed in memory T cells; that is, they expressed CXCR6 and CCR5, which are predominantly expressed on memory CD8+ T cells,10,14,45 as well as granzyme K, which is predominantly expressed in early-stage memory CD8+ T cells.46 Conversely, the CXCR1− cells also expressed the molecules associated with cell lysis and killing, such as perforin, granzyme A, and granzyme B. Therefore, the CXCR1− effector cells have characteristics of both memory precursors and effector cells.
IL-7Rα is reported to be a marker for the transitional effector memory population from the effector pool to memory pool in a mouse model.3 A recent study showed that IL-7Rα+ T cells are derived from the KLRG-1− effector cells having IL-2 productivity, strong cytotoxic activity, granzyme B expression, and productivity for inflammatory cytokines in an acute infection mouse model.5 In the present study, we showed that the CXCR1− subset expressed IL-7Rα and that the CXCR1−IL-7Rα+ subset had substantial cytolytic activity and the highest IL-2 productivity among the 3 subsets of CD27−CD28− effector cells. Therefore, both mouse transitional effector memory cells and human CXCR1−IL-7Rα+ effector CD8+ T cells have the same characteristics of IL-2 productivity and strong cytolytic activity. However, the CXCR1−IL-7Rα+ effector CD8+ T cells may not be transitional effector memory cells, because a high number of CXCR1−IL-7Rα+ effector CD8+ T cells can be detected in individuals who do not have an acute infection. We also discovered that IL-7 up-regulated pSTAT5 in the CXCR1− subset, and promoted their survival, which is consistent with their expression of IL-7Rα. After the contraction phase of an acute infection, virus-specific memory CD8+ T cells are maintained by IL-7 and IL-15 for their survival and proliferation, respectively.47 In healthy individuals, IL-7Rα+ HCMV-specific CD8+ T cells, which contain effector CD8+ T-cell subsets, have shown higher proliferative capacity than the IL-7Rα− cells after IL-7 or IL-15 treatment and stimulation with Ag.48 These studies, together with our present findings, suggest that a mechanism of IL-7 signaling similar to that found in memory CD8+ T-cell homeostasis potentially plays an important role in the maintenance of IL-7Rα+ effector CD8+ T cells.
The results of our present study indicate that the human effector CD8+ T-cell population contains functionally distinct subsets distinguished by their expression of CXCR1 and IL-7Rα. CXCR1+IL-7Rα− effector CD8+ T cells are thought to immediately migrate toward inflammatory sites to exert effector function and then die after they have played their roles. CXCR1−IL-7Rα+ and CXCR1−IL-7Rα− cells possess both effector functions and IL-2 productivity to proliferate themselves, although the former cells have much more IL-2 productivity and IL-7–driven survival capacity, suggesting that they are intermediate between effector T cells and effector memory T cells. The differentiation pathway of these cells remains unclear. IL-2–producing IL-7Rα+ effector CD8+ T cells may be involved in the homeostasis of effector T cells by maintaining the number of effector T cells in the periphery. If this is the case, then this type of effector CD8+ T cell would be essential for protective immunity against viral infections. Further analysis of these effector CD8+ T cells is expected to clarify the differentiation pathway of human CD8+ T cells and the homeostasis of effector T cells. The present study provides new insights into the pathogenesis and development of useful therapy for infectious diseases such as HIV-1 and hepatitis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sachiko Sakai and Yukako Arimura for secretarial assistance and Masumichi Saito and Takamasa Ueno for helpful discussions.
This research was supported by the Global COE program Global Education and Research Center Aiming at the control of AIDS and by grants from the program Grants-in-Aid for Scientific Research (to M.T.) and Young Scientists Start-up (to H.T.) from the Ministry of Education, Culture, Sports, Science, and Technology of the government of Japan. H.T. was a Research Fellow of the Japan Society for the Promotion of Science.
Authorship
Contribution: H.T. and M.T. designed the research; H.T. and T.N. performed the research under the supervision of M.T.; H.T., T.N., and M.T. analyzed the data; and H.T. and M.T. prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Masafumi Takiguchi, Center for AIDS Research, Kumamoto University, 2-2-1 Honjo, Kumamoto 860-0811, Japan; e-mail: masafumi@kumamoto-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal