Abstract
EGFL7 is a secreted angiogenic factor that is highly conserved in vertebrates. Most secreted angiogenic signaling molecules, including VEGF and fibroblast growth factor-2, are mainly expressed by nonendothelial cell types such as fibroblasts. In contrast, EGFL7 is unique because it is almost exclusively expressed by and acts on endothelial cells. Egfl7 expression is highest when the endothelium is in an active, proliferating state. This factor acts as a chemoattractant for endothelial cells and binds to components of the extracellular matrix. In vivo, Egfl7 is important for regulating tubulogenesis in zebrafish and for controlling vascular patterning and integrity in mice. Its function in blood vessel development is mediated, at least in part, through modulation of Notch signaling. In this review, we summarize the findings that support a role for Egfl7 in developmental and postnatal angiogenesis and describe the EGFL7-signaling pathways that underlie these processes. In addition, we discuss a potential role for EGFL7 in vascular repair and its possible use as a therapeutic target for treatment of hypoxia-induced injury. Finally, we consider EGFL7 action during tumorigenesis and its potential as an antiangiogenic agent.
Introduction
The vasculature is a hierarchical system of arteries, arterioles, capillaries, venules, and veins. It provides a conduit that enables the delivery of oxygen and nutrients to cells, and it facilitates the removal of metabolic waste. Embryonic blood vessel formation occurs by 2 processes known as vasculogenesis and angiogenesis.1,2 During vasculogenesis mesoderm-derived cells give rise to endothelial precursors, known as angioblasts. These cells differentiate into endothelial cells and aggregate into cords, forming a primitive vascular plexus. Arterial and venous specificity is also defined during this process.1,2 The vascular plexus is then rapidly expanded by angiogenesis, which involves endothelial cell proliferation and sprouting from preexisting vessels, as well as remodeling of the developing network.1,2 The sprouting vessel comprises 2 distinct endothelial cells: the tip cell and the stalk cell.3 Tip cells are nonproliferative with protruding filopodia that sense and migrate along VEGF gradients.3 In contrast, stalk cells, located behind the tip cell, do not migrate but instead proliferate in response to VEGF, permitting the vascular sprout to elongate away from the parent vessel.3 Sprouting continues in this manner until tip cells of adjacent vessels connect and undergo anastomosis.1,2 On contact, tip cells lose their ability to migrate. This results in the formation of a continuous vessel that consists of a patent lumen, allowing blood flow.4 Mural cells are recruited to the nascent vessel by a process known as arteriogenesis, which in turn stimulates basement membrane deposition and stabilizes the vessel wall.5 The endothelial cells of the resulting neovessel then take on a “phalanx” phenotype, forming a quiescent, tightly aligned endothelial cell layer.1,2 Together, these processes give rise to a functional circulatory system. Such a system is required for tissue growth and regeneration and is also essential for the survival of tumor cells.
Identification of the factors and pathways that are involved in vascular development and regeneration will facilitate the development of novel therapies that could potentially treat vascular disease and injury and lead to strategies to combat cancer. Many molecules have been shown to be crucial for vascular development and neovascularization. For example, VEGF and fibroblast growth factor-2 (FGF-2) are required for endothelial cell differentiation and tube formation.6 The Notch signaling pathway is also essential, controlling various aspects of vascular development, including endothelial cell sprouting and branching.7,8 The importance of miRNAs in the developing and remodeling vasculature has been identified over the past several years. For example, miR126, which is expressed in endothelial cells and highly vascularized tissues,9,10 enhances angiogenesis by repressing negative regulators of the MAPK and PI3K pathways, thereby promoting VEGF and FGF-2 signaling.9-11
Another factor whose importance for vascular development has recently emerged is epidermal growth factor-like domain 7 (Egfl7). In contrast to other secreted angiogenic signaling molecules, Egfl7 is unique because it is predominantly expressed by and acts on endothelial cells.12-17 In this review, we describe the function of Egfl7 in vascular development and summarize the evidence supporting a role for this factor during repair of the vasculature. In addition, we discuss the potential role of Egfl7 in tumorigenesis and its possible use as a therapeutic target for antiangiogenesis therapy.
Egfl7 gene and protein structure
Egfl7, also known as vascular endothelial statin (VE-statin), was identified independently by 3 laboratories as a gene that is expressed in the endothelium during embryogenesis.12-14 The Egfl7 gene has a paralogue known as Egfl8.12,13 Interestingly, an orthologue to the mammalian Egfl8 gene is not present in the fish genome, suggesting that an Egfl7/8 duplication occurred after the fish-mammalian divergence.13
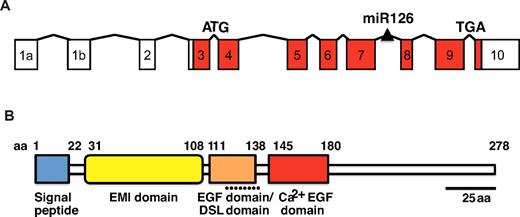
The mouse and human Egfl7 genes are located on chromosome 2 and 9, respectively.12,14 The mouse gene spans 11.5 kb and consists of 11 exons,12,14 with a miRNA, miR126, located between exons 7 and 89-11 (Figure 1A). The 5.4 kb promoter fragment positioned directly upstream of the Egfl7 transcription start site contains 2 evolutionarily conserved regions that consist of consensus Ets binding sites.10 The Ets transcription factor binding sites are sufficient and necessary to drive Egfl7 reporter gene expression in the endothelium throughout embryogenesis and in adult tissues in transgenic mice.10 The Ets binding sites do not, however, comprise a composite FOX/ETS motif that binds the Forkhead and Ets transcription factors and directs expression of endothelial receptors, including VEGF receptor 2 (VEGFR2), Tie2, and Notch4.18 The Egfl7 promoter also contains a GATA-2 consensus binding site, suggesting that GATA transcription factors play a role in controlling expression of the Egfl7 gene.19 ETS and GATA binding sites are common to many genes that are important for early blood and endothelial development.20
Gene structure and protein organization of Egfl7. (A) The Egfl7 gene consists of 11 exons and a miRNA, miR126, embedded in the intronic region located downstream of exon 7. The protein is encoded in exons 3-10 (red boxes). (B) The EGFL7 protein sequence contains several putative protein domains, including a signal peptide (blue box), an EMI domain (yellow box), an EGF domain that contains a Delta-Serrate-LAG-2 (DSL) motif (orange box), and a Ca2+ binding EGF domain (red box).
Gene structure and protein organization of Egfl7. (A) The Egfl7 gene consists of 11 exons and a miRNA, miR126, embedded in the intronic region located downstream of exon 7. The protein is encoded in exons 3-10 (red boxes). (B) The EGFL7 protein sequence contains several putative protein domains, including a signal peptide (blue box), an EMI domain (yellow box), an EGF domain that contains a Delta-Serrate-LAG-2 (DSL) motif (orange box), and a Ca2+ binding EGF domain (red box).
The EGFL7 protein is highly conserved among species. It is contained in exons 3 through 10 and encodes a 29 kDa protein12-14 (Figure 1B). Native and recombinant EGFL7 protein show an apparent mass of ∼ 41 kDa, consistent with posttranslational modification at several potential N- and O-linked glycosylation sites detected in the protein.12 The EGFL7 protein sequence consists of a putative amino-terminal signal peptide domain, an EMI-like domain, and 2 centrally located EGF-like domains12-14 (Figure 1B). Such motifs are commonly found in secreted and extracellular matrix (ECM)–bound proteins.21,22 One of the EGF-like domains comprises a region that is similar to the Delta-Serrate-LAG-2 domain, a sequence that is conserved in ligands that bind Notch,12,23 and the other belongs to a subclass that interacts with Ca2+.12 In support of EGFL7 being secreted, intracellular EGFL7 is localized to organelles of the secretory pathway in cultured cells that overexpress the protein.12,14 EGFL7 is also detected in the cell culture supernatant fluid and is predominantly associated with the ECM after secretion.12,14,17 Together, these data indicate that EGFL7 is a secreted, ECM-bound factor.
Endothelial expression of Egfl7
Egfl7 expression is mostly restricted to the endothelium, with high levels observed in proliferating endothelial cells during embryogenesis and physiologic and pathologic angiogenesis.12-16 During embryogenesis, Egfl7 transcripts are first observed at the 8-cell morula stage.24 In keeping with its endothelial expression, Egfl7 mRNA is detected at sites of both extra-embryonic and embryonic mesodermal progenitors, and in vascular structures that arise by vasculogenesis.12-14 During later stages of organogenesis Egfl7 expression remains associated with cardiovascular structures, albeit at decreased levels.12-14 At this stage the protein exhibits overlapping expression with collagen type IV, consistent with its localization to the ECM.16
EGFL7 is also expressed in the developing postnatal retinal vascular plexus, where it is restricted to sprouting vessels.16,17 The protein is localized to the basal side of stalk cells and shows patchy expression in tip cells in an in vitro–sprouting endothelial cell assay.17 Because of limitations of EGFL7-specific Abs, it is not yet known whether EGFL7 shows a similar pattern in vivo in the retinal vasculature; however, it is conceivable that its expression in vitro will be recapitulated in vivo.
Most adult tissues express EGFL7 in a subset of vessels; however, the levels are considerably lower in quiescent endothelium than in the embryo.13,15 Egfl7 expression is elevated in the endothelium of the pregnant uterus, and increased levels are observed during endothelial regeneration subsequent to vascular injury, suggesting that it plays a role during physiologic and pathologic angiogenesis.13-15 In addition to the endothelium, EGFL7 expression has been detected in progenitor cell populations, megakaryoctyes, and a subset of neurons in the adult brain.12,15,24,25
With the exception of stem cell populations, Egfl7 expression during embryonic development is restricted to the endothelium. Note that all other known secreted angiogenic signaling molecules are expressed not only by endothelial cells but also by a plethora of other cell types. For example, macrophages, epithelial cells, and aortic smooth muscle cells all express VEGF. In fact, endothelial cells are only a minor source of this factor.26 FGF-2 is even more widely expressed and is found in almost all tissues of mesodermal and neuroectodermal origin (http://www.copewithcytokines.org). In this context, EGFL7 is a unique secreted angiogenic-signaling molecule. The similarity of its expression profile to that of the angioblast and endothelial cell marker Vegfr2,27,28 together with its high expression in proliferating endothelium,12-15 suggest that EGFL7 is an excellent candidate marker of endothelial cells and their precursors.
Role of EGFL7 in vascular development
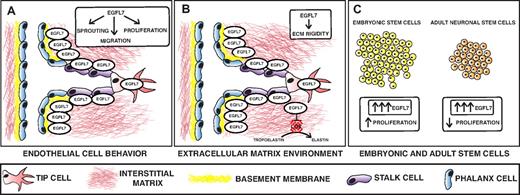
Recent studies suggest that EGFL7 controls blood vessel development by creating a permissive environment for angiogenesis.13,15-17,29 One mechanism by which this occurs is by promoting endothelial cell proliferation, migration, sprouting, and invasion (Figure 2A-B). EGFL7 knockdown in HUVECs suppresses proliferation and migration and inhibits capillary sprouting.16 In addition, EGFL7 is a chemoattractant for embryonic endothelial cells and promotes endothelial cell adhesion.13,15 The adhesion property of EGFL7, however, is weaker than that of other cell-adhesion molecules such as fibronectin and collagens, strengthening the hypothesis that EGFL7 may favor endothelial cell motility.13 Another way in which EGFL7 could promote endothelial cell migration and possibly invasion is by remodeling of the ECM (Figure 2B). It is probable that EGFL7 is secreted by sprouting vessels and deposited in the ECM. In support, EGFL7 expression is localized to endothelial sprouts in the postnatal retinal vasculature.16 Furthermore, EGFL7 deposition to the ECM is facilitated by fibronectin and type I collagen, both of which belong to a group of ECM proteins that are encountered by the nascent vessel on tissue invasion.17 It is conceivable that EGFL7 also modulates the rigidity of the ECM to promote cell migration and invasion. It has been shown that EGFL7 inhibits the deposition of mature elastic fibers by repressing lysyl oxidase (LOX)–mediated conversion of tropoelastin into elastin.29 Thus, it appears that EGFL7 is important in promoting endothelial cell functions that are crucial for angiogenic growth by influencing endothelial cell behavior and signaling to the ECM environment.
Model depicting the role of EGFL7 in angiogenesis and stem cell behavior. (A-B) EGFL7 is a secreted protein that is expressed by endothelial cells, including the stalk and tip cells of sprouting vessels. Once secreted, EGFL7 binds to the ECM and shows overlapping expression with fibronectin and collagen type IV. (A) EGFL7 controls endothelial cell sprouting, migration, and proliferation. (B). EGFL7 also signals to the ECM and modulates its rigidity by inhibiting lysyl oxidase (LOX)–mediated conversion of tropoelastin into elastin. (C) EGFL7 promotes embryonic stem cell proliferation and inhibits proliferation of adult neuronal stem cells.
Model depicting the role of EGFL7 in angiogenesis and stem cell behavior. (A-B) EGFL7 is a secreted protein that is expressed by endothelial cells, including the stalk and tip cells of sprouting vessels. Once secreted, EGFL7 binds to the ECM and shows overlapping expression with fibronectin and collagen type IV. (A) EGFL7 controls endothelial cell sprouting, migration, and proliferation. (B). EGFL7 also signals to the ECM and modulates its rigidity by inhibiting lysyl oxidase (LOX)–mediated conversion of tropoelastin into elastin. (C) EGFL7 promotes embryonic stem cell proliferation and inhibits proliferation of adult neuronal stem cells.
EGFL7 also affects stem cell behavior.25,30 It stimulates embryonic stem cell (ESC) proliferation but inhibits proliferation of adult neuronal stem cells25,30 (Figure 2C). This discrepancy may reflect the difference in multilineage potential between the cell types. EGFL7 does not appear to be required for ESC differentiation into endothelial cells, but it is important for the proper formation of vascular cord structures during ESC differentiation along this lineage.30 In addition, EGFL7 promotes differentiation of adult neuronal stem cells into neurons and oligodendrocytes in vitro by inhibiting Notch.25 EGFL7 may also regulate neuronal differentiation in vivo by modulating the mechanical properties of the ECM. Matrix elasticity directs lineage specification of mesenchymal stem cells.31 In particular, soft matrices are neurogenic.31 Because EGFL7 inhibits elastin deposition, it is conceivable that EGFL7 regulates adult neuronal stem cell differentiation, at least in part, by controlling ECM rigidity.
Recent studies in zebrafish have established a role for egfl7 in vascular development in vivo.13,32 Egfl7 knockdown causes pericardial edema, hemorrhaging, and defects in the circulatory loop.13 The circulatory loop defect is primarily a result of impaired tubulogenesis because the developing vessels in mutant zebrafish display disorganized lumens or, in severe cases, no lumens at all.13,32 In addition, aberrant tight, adherens and gap junctions between membranes are present.13,32 Together, these defects highlight that egfl7 is important for regulating cord-to-tube transition.13,32 The mechanism by which egfl7 regulates tubulogenesis is not yet known. However, it appears to be independent of VEGF signaling because vegf knockdown in zebrafish reduces endothelial cell number and consequently disrupts tube formation, whereas egfl7 knockdown does not alter the total number of endothelial cells.13 In addition to the luminal defects, egfl7 knockdown zebrafish exhibit a reduction in the vascular area extending from the hypochord and the endoderm.32 On the basis of the defects observed, the researchers hypothesized that egfl7 provides a permissive substrate that allows the local movement and proper orientation of angioblasts.13,32 This hypothesis is supported by in vitro studies described earlier which show that EGFL7 regulates endothelial cell migration and adhesion.13,15,16
The role of Egfl7 in mammalian vascular development has been complicated by the presence of miR126.10,11 Two separate Egfl7 loss-of-function mouse models, generated by Schmidt et al, exhibit partial embryonic lethality and vascular abnormalities, including edema and reduced vascular coverage in the head and retina.17 Together, these results suggested that Egfl7 is required for normal vascular development. However, it should be noted that the level of miR126 expression was not reported in the study.17 Kuhnert et al independently generated a third knockout model that is viable and appears phenotypically normal.11 These mice lack Egfl7 expression, but the level of miR126 is unaltered. It has therefore been suggested that the discrepancy between Egfl7 loss-of-function phenotypes should be ascribed to differences in the level of miR126 and not Egfl7.11 In support, selective miR126 knockout in mice results in partial embryonic lethality, edema, and delayed vascular development, similar to what has been described by Schmidt et al17 for Egfl7 knockout mice.10,11 The lack of an overt vascular phenotype in mice with Egfl7-specific loss-of-function that were generated by Kuhnert et al may simply reflect the mixed genetic background in which analysis was performed, the presence of strain-specific genetic modifiers, or both.11 The absence of a phenotype may also be because of compensation by other genes.
A miR126 orthologue is located in the zebrafish egfl7 gene. In view of the controversy surrounding the role of Egfl7 and miR126 in mice, it is important to reconsider the cause of the vascular abnormalities observed in egfl7 knockdown zebrafish, especially because selective miR126 knockdown causes a similar phenotype to egfl7 knockdown.9 However, the observation that injection of morpholino antisense oligonucleotides to target egfl7 results in defects that can be rescued by co-injection of egfl7 mRNA indicates that egfl7 has a defined function in zebrafish vascular development that cannot be compensated by other genes.13
Because murine Egfl7 loss-of-function phenotype is still unresolved, it raises the question of whether Egfl7 has a functional role during mammalian vascular development. Egfl7 transgenic mouse models have been useful tools in addressing this issue. Overexpression of Egfl7 in mice results in abnormal patterning and remodeling of blood vessels.16 Transgenic mice that overexpress endothelial Egfl7 mRNA by 2-fold, without altering miR126 levels (Tie2-Egfl7 transgenic mice), exhibit partial embryonic lethality, which is accompanied by hemorrhaging and abnormal vascular patterning. Specifically, abnormalities in the embryonic head vasculature include a decrease in the number of major cranial vessels, abnormal endothelial cell aggregates, and collapsed arterial vessels. The yolk sac vasculature exhibits increased vessel caliber and knot-like venous structures. Vessel defects are also observed in the postnatal retinal vasculature and include excessive arterial branching and tortuous veins. In addition, overexpressing Egfl7 cDNA causes a subtle retinal hyperangiogenic response, which results in increased vascular coverage and an elevated number of sprouting filopodia in the peripheral plexus.16 Tie2-Egfl7 transgenic mice also exhibit vascular stratification defects.16 A different transgenic mouse model, in which EGFL7 is ectopically expressed in the epidermis with the use of the human keratin-14 promoter, exhibits defects in arterial elastogenesis, suggesting that EGFL7 also contributes to vessel stability.29 Together, the phenotypes observed for the Egfl7 transgenic mouse models indicate that this factor is important for mammalian blood vessel formation.
In summary, Egfl7 controls blood vessel development by promoting endothelial cell migration, proliferation, sprouting, adhesion, and possibly invasion. Altered levels of this factor result in abnormal vessel patterning, remodeling, and stabilization, highlighting the importance of EGFL7 in these processes.
EGFL7 in vascular injury and disease
EGFL7 appears to also play a role in the repair of the microvasculature in response to vascular damage. In support, Egfl7 expression is temporarily induced in regenerating endothelium of mice that have been subjected to arterial vascular insult.15 Vascular injury is often associated with hypoxic environments. Accordingly, increased levels of Egfl7 are detected in response to hypoxia in both cultured endothelial cells and in the immature rat brain in vivo.33,34 The mechanism by which Egfl7 expression is up-regulated in response to hypoxia is unknown. Note that the Egfl7 promoter contains 9 potential hypoxia-inducible factor-1α binding sites, and it is, therefore, possible that induction of Egfl7 expression is mediated by this transcription factor directly binding to and transactivating the Egfl7 promoter.
Recent studies suggest a protective role for EGFL7 against vascular injury and ischemia. An increase in the level of Egfl7 under hypoxic conditions appears to stimulate an angiogenic response. Indeed, elevated Egfl7 expression in response to hypoxia in the neonatal rat brain is accompanied by increased vascular density.34 Interestingly, this appears to convey a neuroprotective effect against hypoxic-ischemic insult.34 EGFL7 may also stimulate vascular regeneration by repressing key steps in the inflammatory activation of endothelial cells.33,35 Specifically, it has been shown that EGFL7 represses NFκB activation and the subsequent expression of ICAM-1 in response to hypoxia/reoxygenation-induced and calcineurin inhibition–induced endothelial injury.33,35
In contrast to hypoxic conditions, EGFL7 expression is inhibited after hyperoxic exposure in both neonatal lungs in vivo and in endothelial cells in vitro, and reduced levels are associated with endothelial cell death.36 In support, EGFL7 overexpression in endothelial cell lines results in a protective effect against hyperoxia-induced apoptosis by preventing cytochrome c release, inhibiting caspase-3 activation, and preventing the induction of BAX expression, all of which are hallmarks of mitochondria-induced apoptosis.36
The studies reviewed in this section indicate that EGFL7 may potentially be used as a future therapeutic target to treat diseases associated with hypoxic-ischemic insult, including atherosclerosis, coronary artery disease, and stroke. In addition, EGFL7 therapy may be useful in preventing endothelial cell damage in patients who require long-term, high oxygen treatment or exposure, such as premature infants and patients being treated for pneumonia.37
EGFL7 and the Notch signaling pathway
Recently, progress has been made in understanding the molecular mechanisms that underlie Egfl7-mediated control of blood vessel development. With the use of the transgenic mouse model in which Egfl7 is overexpressed in endothelial cells, Nichol et al showed that EGFL7 regulates this process, at least in part, by modulation of Notch signaling.16 Because it is bound to the ECM, EGFL7 probably exerts its effects on vascular development by signaling to Notch receptors on endothelial cells. This could be achieved in an autocrine manner, whereby EGFL7 is secreted from the same endothelial cell that it signals to, or it may be achieved in a paracrine manner by signaling to Notch receptors on neighboring cells. It is also possible that EGFL7 will signal to the surrounding perivascular cells and enhance vessel maturation. Such a role has been suggested for the Notch ligand, Jagged1.38 In support, the vascular stratification defects that were observed in Tie2-Egfl7 transgenic embryos indicate that EGFL7 affects the spatial organization of endothelial and mural cells.16
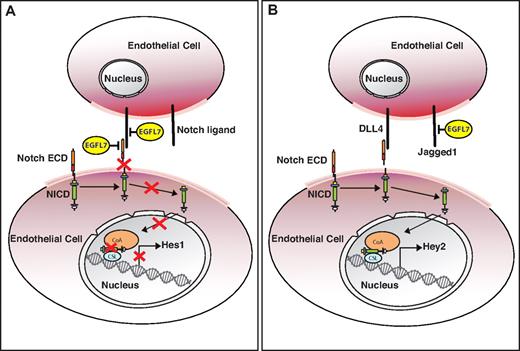
EGFL7 overexpression in the postnatal retina reduces Notch target gene expression and induces a subtle hyperangiogenic response, similar to what has been observed when Notch signaling is inhibited.39-42 This suggests that Egfl7 acts as a Notch antagonist. In support, Egfl7 knockdown in HUVECs results in inhibition of endothelial cell proliferation, new sprout formation, and migration.16 These cellular responses are also observed when Notch signaling is activated in human cultured endothelial cells.43-46 Furthermore, overexpression of EGFL7 represses Notch reporter activity and inhibits Jagged1/Jagged2-mediated Notch activation in vitro.16,25 EGFL7 interacts with the endothelial Notch receptors Notch1 and Notch4 and their ligand, DLL4.16,25 This indicates that EGFL7 could inhibit Notch signaling by binding to the Notch receptor or its corresponding ligand, resulting in blocking ligand/receptor interaction (Figure 3A). In contrast to the postnatal retina, some Notch target transcripts are up-regulated in Tie2-Egfl7 embryos, suggesting that EGFL7 may stimulate Notch activity prenatally.16 Although the underlying mechanism for Notch activation by EGFL7 remains unknown, some clues have emerged from studies that used primary HUVECs. Reporter gene assays performed in HUVECs that either overexpress or exhibit knockdown levels of EGFL7 indicate that EGFL7 represses Notch reporter activity.16,25 However, when reporter assays were performed in the presence of DLL4, EGFL7 appears to enhance Notch signaling.25 Specifically, EGFL7 knockdown in HUVECs inhibits Notch signaling when the cells are seeded on DLL4-coated plates.25 This result is consistent with EGFL7 mediating DLL4/Notch signaling indirectly by binding to factors that inhibit activation of this pathway. This is relevant in the context of the glycoslyation status of Notch. Glycosylation of Notch receptors by Fringe family glycosyltransferases enhances DLL4/Notch signaling and weakens the signaling capacity of Jagged1.39 Under such circumstances Jagged1 competes with DLL4 for Notch binding and acts as an antagonist of this signaling pathway.39 Although EGFL7 does not bind to Jagged1 in an in vitro yeast-2-hybrid assay, this does not preclude an interaction in vivo.25 It is conceivable that EGFL7 binding to Jagged1 when the Notch receptor is glycosylated will prevent an interaction between the receptor and Jagged1, and in turn promote DLL4/Notch binding, thus activating Notch signaling (Figure 3B). This would provide an explanation for how EGFL7 overexpression could stimulate the Notch pathway in the embryo.
Model describing the putative role of EGFL7 in Notch signaling in different vascular beds. (A) EGFL7 signaling in the postnatal retina. EGFL7 antagonizes Notch/ligand interaction by either binding to the receptor or its corresponding ligand and inhibits target gene expression. (B) EGFL7 signaling in the embryo. EGFL7 indirectly enhances DLL4/Notch signaling by binding to Jagged1, preventing an interaction with the glycosylated Notch receptor, and promoting DLL4 interaction with Notch. ECD indicates extracellular domain; and NICD, Notch intracellular domain.
Model describing the putative role of EGFL7 in Notch signaling in different vascular beds. (A) EGFL7 signaling in the postnatal retina. EGFL7 antagonizes Notch/ligand interaction by either binding to the receptor or its corresponding ligand and inhibits target gene expression. (B) EGFL7 signaling in the embryo. EGFL7 indirectly enhances DLL4/Notch signaling by binding to Jagged1, preventing an interaction with the glycosylated Notch receptor, and promoting DLL4 interaction with Notch. ECD indicates extracellular domain; and NICD, Notch intracellular domain.
The studies described in this section highlight the ability of EGFL7 to elicit both agonistic and antagonistic effects on Notch signaling. To reconcile these findings, we hypothesize that the environment within the vascular bed plays an important role in how EGFL7 affects the Notch pathway. For example, it is probable that the presence of EGFL7 in a secreted or ECM-bound form, the availability of Notch receptors and ligands, and the posttranslational modifications of Notch will affect the EGFL7/Notch interaction.
Gene inactivation and overexpression studies in mice have established that Notch signaling is essential for the reorganization of vessels during angiogenesis.7 Altered Notch signaling affects several aspects of angiogenesis, including sprouting, branching, and proliferation of the vascular network.7,8 Studies in mice that overexpress endothelial EGFL7 show that this factor is also important for vessel patterning and remodeling.16 However, the phenotypes observed in the Tie2-Egfl7 transgenic embryos do not completely recapitulate other Notch gain-of-function mutants.47,48 Namely, dilation and arterialization of vessels were not observed.16,47,48 Likewise, the retinal phenotype is subtler than one would expect for Notch loss-of-function.16 These findings suggest that EGFL7 acts to “fine-tune” Notch signaling, possibly by competing with other Notch ligands for receptor binding or by interacting with the ligands directly (Figure 3). Although the studies described have established that EGFL7 modulates Notch signaling in vitro and in vivo, further analysis is required to address the exact mechanism of EGFL7 and Notch interaction and the precise role of this signaling pathway during vascular development.
EGFL7 and tumorigenesis: a potential therapeutic target for the treatment of cancer
EGFL7 is expressed in several tumors and many cancer cell lines.13,49-51 EGFL7 expression is highly elevated in human tumors, including kidney tumors, malignant gliomas, hepatocellular carcinomas (HCCs), and colon cancers.13,49-51 Because endothelial EGFL7 overexpression in mice results in abnormal vessel patterning and remodeling,16 it is possible that EGFL7 stimulates tumor angiogenesis and contributes to the irregularly shaped, tortuous, leaky vessels that are characteristic of the tumor vasculature, in a similar manner to VEGF.52 In gliomas, EGFL7 expression correlates with increased cell proliferation and microvascular density.50 Interestingly, in this type of brain cancer EGFL7 expression is localized to both tumor cells and the surrounding vasculature.50 Therefore, it is possible that EGFL7 exerts its effect on the vasculature either in an autocrine manner, a paracrine fashion, or by cross talk between the 2 cell types.
Injection of mice with HCC cells that express knockdown levels of EGFL7 results in the formation of smaller tumors that exhibit reduced vascular density compared with the tumors formed in mice that were injected with control cells,51 further supporting that EGFL7 regulates tumor angiogenesis. In this instance, EGFL7 appears to signal to the vasculature in a paracrine manner because its expression is mainly restricted to HCC cells.51 One mechanism by which this may occur is by activating Notch signaling. Blocking EGFL7, at least in HCC cells, does not lead to the overgrowth of blood vessels that has been observed when the DLL4/Notch pathway is impaired in tumors.53-55 Instead, inhibiting EGFL7 signaling is reminiscent of the reduction in tumor angiogenesis that is seen when Notch signaling is blocked with the use of a soluble form of the Notch1 receptor (Notch 1 decoy).56
Several studies have shown the benefits of blocking Notch signaling in tumor models.53-56 For example, DLL4 blockade in mice with the use of a neutralizing anti-DLL4 Ab or recombinant DLL4 protein inhibits the growth of several solid tumors.53-55 In addition, blocking Notch signaling with the use of the Notch1 decoy disrupts human and mouse tumor xenograft growth after implantation into mice.56 On the basis of these data, members of the Notch signaling pathway may be considered as promising therapeutic drug targets in the treatment of cancer. However, chronic suppression of Notch in mice, by either blocking DLL4 or using a mouse model of sporadic Notch1 loss of heterozygosity, results in adverse side effects, including disorders in multiple organs and the development of vascular tumors.57,58 Therefore, if EGFL7 does function as a Notch agonist during tumorigenesis, the long-term effect of EGFL7 blockade will have to be taken into consideration. Interestingly, the liver is the organ that exhibits the highest number of vascular neoplasms in response to a block in Notch signaling.57,58 Note that Egfl7 expression is elevated in the livers of mice when Notch signaling is blocked.58 Thus, it will be important to determine whether Egfl7 overexpression is responsible, at least in part, for the adverse side effects caused by Notch inhibition.
The elevated levels of EGFL7 expression in tumors and its possible role in promoting tumor angiogenesis make EGFL7 a potential target for antiangiogenesis therapy. The concept of antiangiogenic strategies is to deprive the tumor of essential nutrients by inhibiting new vessel growth and destroying existing ones.52 One example is anti-VEGF treatment, which combined with chemotherapy has shown to be of clinical benefit in the context of solid tumors.59 Anti-VEGF therapy prunes preexisting vessels but also inhibits the growth of new ones.52 In addition, it induces vessel normalization by increasing mural cell recruitment and vessel maturation.60 The resulting normalized tumor vasculature improves the delivery of cytotoxic drugs. However, this improvement is transient because prolonged VEGF blockade ultimately prunes most of the vessels, including the normalized ones.60
Recent preclinical studies that used a combination of anti-VEGF therapy and humanized monoclonal Abs against EGFL7 provided additional inhibition of tumor growth in human cancer xenograft mouse models, compared with anti-VEGF treatment alone (http://ip.com/patapp/US20110200602). Currently, clinical trials are being conducted to evaluate the effect of combined anti-EGFL7 and Avastin (anti-VEGF Ab) therapy on tumor vascular function and growth (http://www.gene.com/gene/pipeline/status/oncology/anti-egfl7). It is expected that blocking EGFL7 signaling in conjunction with anti-VEGF will provide additional inhibition of angiogenesis. With respect to the role of EGFL7 in vascular remodeling and patterning during development, anti-EGFL7 therapy may also induce normalization of the vasculature which, when used together with anti-VEGF treatment, could extend the window for improved drug delivery. Although the potential for anti-EGFL7 therapy is promising, the long-term outcome and its possible effect on Notch signaling must also be evaluated.
In addition to its role in tumor angiogenesis, EGFL7 has been shown to enhance intrahepatic and pulmonary metastasis of HCC cells by promoting cell motility by EGF receptor-dependent phosphorylation of focal adhesion kinase.51 In support of a general role in other tumor metastasis, EGFL7 overexpression in breast and lung carcinoma cells leads to an increase in metastasis,61 and high EGFL7 expression is associated with lymph node metastasis in colon cancer.49 Importantly, levels of EGFL7 expression significantly correlate with pathologic characteristics associated with clinical progression, poor prognosis, and tumor grade in a variety of tumors, including malignant gliomas, HCC, and colon tumors.49-51 Therefore, EGFL7 may be suitable as a predictive factor for the clinical progression of cancer and might prove useful as a therapeutic target to prevent metastasis.
A recent study shows that EGFL7 may also indirectly promote tumor growth by protecting tumors from a host immune response.61 Specifically, Egfl7 overexpression in breast and lung carcinoma cells enhances tumor progression in immunocompetent mice and appears to do so by reducing the expression of tumor endothelial leukocyte adhesion molecules, including ICAM-1 and VCAM-1, thus preventing host immune cell infiltration.61 The repression of ICAM-1 expression by EGFL7 is in keeping with observations made during endothelial cell injury33,35 and highlights a possible novel mechanism by which EGFL7 promotes tumorigenesis.
Because of its potential to promote tumor growth and metastasis, it is tempting to speculate that EGFL7 may prove a promising therapeutic target in the treatment of cancer.
Conclusion
EGFL7 is a secreted protein that is expressed by and acts on endothelial cells to control blood vessel development during both physiologic and pathologic angiogenesis. Its function in vascular development is, at least in part, mediated by modulating Notch signaling. Its high expression in proliferating endothelium and its role in promoting angiogenesis highlight the promise of EGFL7 as a therapeutic target to combat cancer and to treat vascular diseases.
Acknowledgments
The authors thank members of the Stuhlmann laboratory, in particular Kathryn Bambino and Lauretta Lacko, for critically reading and editing the manuscript. They thank David Nichol (Creatavision) for the illustrations.
This work was supported by the National Institutes of Health (grant RO1HL0820898, H.S.), a Tri-Starr Stem Cell Scholar Postdoctoral Fellowship (D.N.), and the Weill Cornell Training Program in Stem Cell Biology and Regenerative Medicine (NYSTEM) Postdoctoral Fellowship (D.N.).
National Institutes of Health
Authorship
Contribution: D.N. and H.S. co-wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Heidi Stuhlmann, PhD, Department of Cell and Developmental Biology, Weill Cornell Medical College, 1300 York Ave, Box 60, New York, NY 10065; e-mail: hes2011@med.cornell.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal