Abstract
The International Immune Tolerance Study was a multicenter, prospective, randomized comparison of high-dose (HD; 200 IU/kg/d) and low-dose (LD; 50 IU/kg 3 times/week) factor VIII regimens in 115 “good-risk,” severe high-titer inhibitor hemophilia A subjects. Sixty-six of 115 subjects reached the defined study end points: success, n = 46 (69.7%); partial response, n = 3 (4.5%); and failure, n = 17 (25.8%). Successes did not differ between treatment arms (24 of 58 LD vs 22/57 HD, P = .909). The times taken to achieve a negative titer (P = .027), a normal recovery (P = .002), and tolerance (P = .116, nonsignificant) were shorter with the HD immune tolerance induction (ITI). Peak historical (P = .026) and on-ITI (P = .002) titers were correlated inversely with success, but only peak titer on ITI predicted outcome in a multivariate analysis (P = .002). LD subjects bled more often (odds ratio, 2.2; P = .0019). The early bleed rate/month was 0.62 (LD) and 0.28 (HD; P = .000 24), decreasing by 90% once negative titers were achieved. Bleeding was absent in 8 of 58 LD versus 21 of 57 HD subjects (P = .0085). One hundred twenty-four central catheter infections were reported in 41 subjects (19 LD); infection frequency did not differ between the treatment arms. Neither bleeding nor infection influenced outcome. Although it was stopped early for futility and safety considerations, this trial contributed valuable data toward evidence-based ITI practice.
Introduction
The only proven strategy for achieving Ag-specific tolerance to factor VIII (FVIII) is immune tolerance induction (ITI). Successful ITI leads to normalization of FVIII pharmacokinetics with consequent improvement in the patient's quality of life. Our current knowledge about ITI in severe hemophilia A is derived from small cohort studies1-6 and retrospective national and international ITI registries.7-9 ITI success rates of 53%-79% have been reported.10
ITI outcome is influenced by host- and treatment-related variables. The International Immune Tolerance Registry (IITR), the German Registry, and the North American Immune Tolerance Registry (NAITR) have all identified parameters of the host immune response to FVIII that influence outcome. Lower pre-ITI (< 10 Bethesda units [BU]), historical peak, and peak titers on ITI were all strongly correlated with ITI success and time to success.7-9 Conversely, ethnicity and F8 genotype have not been shown to affect outcome.9,11,12
Potential treatment-related ITI outcome variables include bleeding, central venous catheter device (CVAD) infection, FVIII type, and dosing regimen. Although CVAD infections have been reported repeatedly to adversely affect ITI outcome,13-18 they did not predict ITI outcome in the NAITR.9 The impact of bleeding has never been examined.
A retrospective analysis of the Frankfurt experience suggested a better outcome when plasma-derived, VWF-containing FVIII was used19 However, this was not confirmed by either the IITR or the NAITR,7,9 and tolerance has been achieved using either recombinant or plasma-derived FVIII.20-27 Furthermore, successful ITI with VWF-containing FVIII products may be influenced by Ab epitope specificity.28-30
The role of FVIII dosing regimen in ITI has generated the greatest debate despite the absence of definitive data. Doses between 50 IU FVIII/kg 3 times weekly and 300 IU/kg/d have been used with comparable results.1-9 The IITR and NAITR generated conflicting data.7,9 In the IIITR, FVIII doses of ≥ 200 U/kg/d resulted in greater success,31 whereas the NAITR reported an inverse relationship between FVIII dose and ITI success.9 A meta-analysis of both registries determined that, for patients with historical inhibitor titers < 200 BU and immediate pre-ITI titers < 10 BU, outcome was uninfluenced by dose.32 These parameters have therefore been used to define a “good-risk” subgroup of ITI patients. Other predictors of better outcome, including younger age at ITI initiation,7,9 an interval < 5 years between inhibitor diagnosis and ITI start,7,9 and ITI interruption < 2 weeks in duration,8 further define a good-risk subset of patients undergoing ITI.
Although ITI practice guidelines were developed on the basis of low-level published evidence,33-36 in the absence of prospective controlled trials, the optimum ITI dosing regimen has never been established. Broader availability of ITI may require a better understanding of the relative efficacy, morbidity, and cost effectiveness of current ITI regimens.
We present herein the principal results of a randomized, controlled comparison of high-dose (HD) and low-dose (LD) immune tolerance induction in a good-risk cohort of severe hemophilia A high-titer inhibitor subjects. This study was designed to test the hypothesis that overall response to ITI is independent of FVIII dosing regimen in good-risk subjects. The study also investigated several questions raised in the literature about potential predictors of ITI outcome and morbidity of ITI.
Methods
Subjects
Subjects with severe hemophilia A (FVIII < 0.01 IU/mL) were recruited from 70 centers in 17 countries between July 1, 2002 and November 13, 2009, when the study was terminated. The inclusion criteria are summarized in Table 1. Participating centers also submitted basic data on inhibitor subjects not recruited to the ITI study so that we could discover the reasons for nonrecruitment and detect recruitment bias.
Study inclusion criteria
|
|
Study
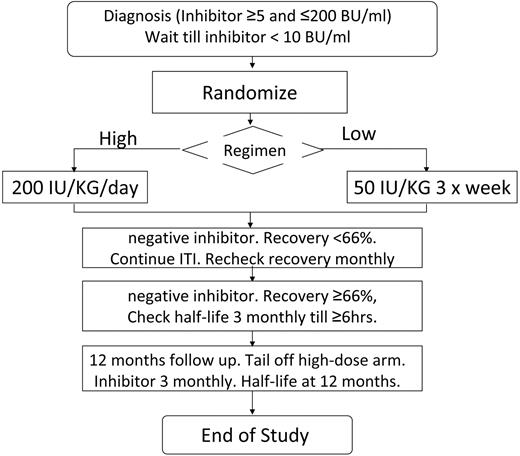
Subjects were recruited as soon as possible after inhibitor detection and inhibitors were checked locally every 4 weeks. The protocol specified that bleeding should be treated with nonanamnestic bypass therapy before ITI. The study plan is summarized in Figure 1. The study was approved by the institutional review boards of all participating hospitals. Written informed consent was obtained in accordance with the Declaration of Helsinki. Patients were computer randomized using the method of minimization, minimizing for product type (recombinant or plasma derived) and starting inhibitor titer above/below 5 BU/mL. Randomization was deferred until the inhibitor titer had decreased below 10 BU/mL. Subjects were randomized to ITI with either 50 IU/kg of FVIII 3 times weekly (LD) or 200 IU/kg of FVIII daily (HD). FVIII dose compliance was monitored and a 20% variance from the total ITI dose was permitted.
Flow diagram summarizing the protocol for the study, randomization, and monitoring of ITI and prophylaxis.
Flow diagram summarizing the protocol for the study, randomization, and monitoring of ITI and prophylaxis.
The choice of recombinant FVIII (rFVIII) or plasma-derived FVIII (pdFVIII) brand used for ITI and the use and management of central venous access devices (CVADs) was left to the discretion of the managing clinician. Switching of FVIII brands during the course of ITI was not permitted. Immune tolerance continued for a minimum of 9 and a maximum of 33 months. FVIII used in the study was supplied through each country's national or regional health service. FVIII subsidies were negotiated with suppliers for subjects in the United States, Japan, and Argentina. In the United States, subsidized clotting factor was distributed to participating subjects through Gulf States Pharmacy, University of Texas Medical School at Houston.
After starting ITI, inhibitor measurements were conducted locally once monthly by either the Bethesda or Nijmegen modification assay. Once the inhibitor had been confirmed to be negative, FVIII recovery was measured monthly after an infusion of 50 U/kg of FVIII without a washout period. Once FVIII recovery was shown to be ≥ 66% of expected, a FVIII half-life study was conducted using 50 U/kg of FVIII after a 3-day treatment-free washout period and was repeated, if necessary, every 12 weeks until the half-life was ≥ 6 hours. Half-life was calculated centrally using Win-Non-Lin Version 2.1 software (Pharsight) from values obtained before infusion and 15-30 minutes, and 1, 2, 4, 6, 24, and 48 hours after infusion.
The study definitions of successful tolerance, partial response, study failure, and relapse are described in Table 2.
Study definitions of successful tolerance, partial response study failure, and relapse
| Successful tolerance . | Negative inhibitor titer, FVIII recovery ≥ 66% of expected, and FVIII recovery ≥ 6 h . |
|---|---|
| Partial response | After 33 mo of ITI, negative inhibitor titer but persistently abnormal recovery or half-life; responding clinically to FVIII replacement without an anamnestic increase in inhibitor titer |
| Study failure | Failure of the inhibitor to decline by ≥ 20% over any 6-mo period after the first 3 mo of immune tolerance induction (ITI); or failure to achieve tolerance or partial response after 33 mo on ITI; or withdrawal from the study for any reason before tolerance was achieved |
| Relapse | Inhibitor recurrence during the 12-mo follow-up period on prophylaxis after tolerance was achieved, as evidenced by recurrent positive Bethesda titer or a decline in FVIII recovery or half-life below study limits |
| Successful tolerance . | Negative inhibitor titer, FVIII recovery ≥ 66% of expected, and FVIII recovery ≥ 6 h . |
|---|---|
| Partial response | After 33 mo of ITI, negative inhibitor titer but persistently abnormal recovery or half-life; responding clinically to FVIII replacement without an anamnestic increase in inhibitor titer |
| Study failure | Failure of the inhibitor to decline by ≥ 20% over any 6-mo period after the first 3 mo of immune tolerance induction (ITI); or failure to achieve tolerance or partial response after 33 mo on ITI; or withdrawal from the study for any reason before tolerance was achieved |
| Relapse | Inhibitor recurrence during the 12-mo follow-up period on prophylaxis after tolerance was achieved, as evidenced by recurrent positive Bethesda titer or a decline in FVIII recovery or half-life below study limits |
From the consensus proceedings from the Second International Conference on Immune Tolerance Therapy, Bonn, Germany, 1997 (unpublished).
Once tolerance was achieved, subjects were changed to prophylaxis using 30 IU/kg of FVIII 3 times weekly, and were monitored for inhibitor recurrence for a further 12 months. HD subjects were tapered down to this dose over 3 months. Subjects were monitored quarterly using inhibitor titer and FVIII recovery measurements. A FVIII half-life was also requested at the end of the study. Patients were managed and centralized decisions reached on the basis of local inhibitor determinations. Critical inhibitor measurements such as starting titer and first negative titer were confirmed centrally by The Hematology Laboratory, Radboud University Nijmegen Medical Center, The Netherlands using the Nijmegen modification of the Bethesda assay with a lower limit of detection of 0.2 BU/mL.
Data handling and statistical analysis
Data were collected electronically. Clotting factor usage, intercurrent bleeding, surgery, hospitalizations, concomitant medications, all infections, and any other adverse events were documented prospectively. For the purpose of analysis, ITI was divided into 4 phases: phase 1 was considered to be the time from the start of ITI until the inhibitor titer was negative; phase 2 was the time from end of phase 1 until the FVIII recovery was ≥ 66% of expected; phase 3 was the time from the end of phase 2 until tolerance was achieved; and Phase 4 was the 12-month period of prophylaxis post-tolerance induction.
Statistics were conducted at the Christie Hospital and Patterson Institute Clinical Trials Unit using SPSS Version 16 and S-Plus 2000 software. The Mann-Whitney U test was used for all 2-arm comparisons. Cumulative bleeds over time were analyzed using the Cox frailty model and survival times were analyzed using the log-rank test. The relationship between variables and the outcome of ITI was analyzed by logistic regression.
The original power calculation, based on the literature, indicated that a sample of 75 subjects per treatment arm would have 80% power at the P = .05 significance level by 2-sided log-rank test to detect a treatment arm difference if 80% of the HD group and 50% of the LD group achieved tolerance after 9 months of ITI. This calculation assumed the loss of up to 5 subjects per treatment arm and 2 interim analyses.
Serious adverse events (SAEs) and 2 interim safety and efficacy analyses were adjudicated prospectively by an independent data safety monitoring board (DSMB) following a DSMB protocol. Interim analyses were conducted when 50 and 100 subjects had reached a primary study end point for the evaluation of the DSMB, with investigators remaining blinded.
Results
Subject accrual and demographics
At the time of study closure, 134 subjects were enrolled (Figure 2). An additional 171 patients managed by 55 centers not recruited to the study were reported to us, of whom 161 were ineligible based on one or more subject exclusion criteria. Ten otherwise eligible subjects were not recruited because of clinician concerns about randomization.
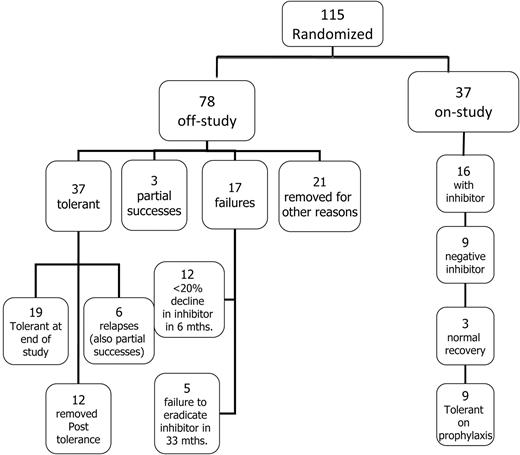
Flow diagram showing the disposition of all 115 patients randomized and started on ITI.
Flow diagram showing the disposition of all 115 patients randomized and started on ITI.
Ten of 134 enrolled subjects were removed from the study before randomization: 4 took longer than the prescribed limit for the inhibitor to decline to < 10 BU/mL, the inhibitor titer rose to > 200 BU/mL in 1 subject, consent was withdrawn in 4 subjects, there was 1 death from a traumatic intracerebral bleed, and 8 subjects had not been randomized at the time of study termination.
Although 116 subjects were randomized, 1 was withdrawn by the investigator without starting ITI and was therefore excluded from this analysis. Ultimately, 115 subjects started ITI at a median age of 15.5 months (IQR, 11.0-24.0; 58 LD and 57 HD; Figure 2). Of these, 108 (94%) were randomized sufficiently before study termination to have sufficient data for analysis of therapeutic efficacy and safety. Randomized subject demographics did not differ between the treatment arms (Table 3).
Demographics for 115 randomized subjects by treatment arm reported as median (IQR) except as stated
| . | LD (n = 58) . | HD (n = 57) . | P . |
|---|---|---|---|
| Randomization age, mo | 15.6 (10.7-23.2) | 14.4 (11.4-25.3) | .75 |
| Diagnostic inhibitor titer, BU/mL | 9.9 (5.0-18.2) | 11.5 (6.3-23.0) | .52 |
| Peak historical inhibitor titer, BU/mL | 21.7 (13.4-52.5) | 22.4 (12.5-50.0) | .78 |
| Inhibitor titer at randomization, BU/mL | 5.9 (3.3-7.3) | 5.1 (3.0-7.4) | .85 |
| Peak inhibitor titer on ITI, BU/mL* | 40.1 (7.6-150) | 33.0 (1.5-205) | .37 |
| Total time on ITI, mo* | 16.4 (10.5-22.4) | 14.2 (9.1-22.6) | .70 |
| Ethnicity, n† | |||
| White | 32 | 27 | |
| African | 5 | 4 | |
| Asian | 12 | 11 | |
| Other | 9 | 14 | |
| . | LD (n = 58) . | HD (n = 57) . | P . |
|---|---|---|---|
| Randomization age, mo | 15.6 (10.7-23.2) | 14.4 (11.4-25.3) | .75 |
| Diagnostic inhibitor titer, BU/mL | 9.9 (5.0-18.2) | 11.5 (6.3-23.0) | .52 |
| Peak historical inhibitor titer, BU/mL | 21.7 (13.4-52.5) | 22.4 (12.5-50.0) | .78 |
| Inhibitor titer at randomization, BU/mL | 5.9 (3.3-7.3) | 5.1 (3.0-7.4) | .85 |
| Peak inhibitor titer on ITI, BU/mL* | 40.1 (7.6-150) | 33.0 (1.5-205) | .37 |
| Total time on ITI, mo* | 16.4 (10.5-22.4) | 14.2 (9.1-22.6) | .70 |
| Ethnicity, n† | |||
| White | 32 | 27 | |
| African | 5 | 4 | |
| Asian | 12 | 11 | |
| Other | 9 | 14 | |
Data for 108 subjects.
Ethnicity data missing for 1 subject.
Among the 115 randomized subjects, the median time for the inhibitors to decline to < 10 BU/mL from the time of first detection was 5.5 months (IQR, 3.1-8.4). At the time of study termination, 37 of 115 (31%) randomized subjects remained in the study, 9 of whom were tolerant but still in the prophylaxis phase of the study. (Figure 2). Seventy-eight (68%) of 115 randomized subjects had reached an off-study end point at the time of study termination (Figure 2); 37 achieved tolerance, 3 met the criteria for partial success, and 17 failed ITI (Figure 2).
Twenty-one (18%) randomized subjects were withdrawn for other reasons before reaching a defined study end point; 8 were withdrawn by physicians or parents, 12 were withdrawn for poor compliance or major protocol violations such as a major dose change, and 1 was lost to follow-up after tolerance (Figure 2). Withdrawals were balanced by treatment arm: 12 HD and 9 LD subjects were withdrawn after a median of 19 (IQR, 9-27) and 15 (IQR, 9-17) months, respectively. Twelve subjects were lost to follow-up during the prophylactic phase: 8 because of failure to comply or gross protocol deviation (most commonly ceasing regular follow-up), 2 were withdrawn by the parent or investigator, and 2 were lost to follow-up. Losses after tolerance were also balanced by treatment arm: 6 HD and 6 LD after a median of 16.5 (IQR, 8.8-30) and 26.5 (IQR, 24-32) months of treatment, respectively.
rFVIII was used for ITI in 102 of 115 subjects (90%). Product type was equally distributed between treatment arms (rFVIII in 52 LD and 50 HD; pdFVIII in 7 of 13 HD and 6 LD subjects).
FVIII dose compliance met the study requirement for 89 of 108 (82%) subjects for whom we had sufficient follow-up for analysis. Four LD subjects exceeded the required dose by a median of 32%, and 15 (12 HD) received a median of 49% less FVIII than that required by the protocol.
Between 0 and 4 confirmatory samples were requested from each subject, depending on how far through the study the patient had progressed. In the end, 139 of 160 (86.8%) confirmatory samples were retrieved for central testing from 63 of 115 randomized subjects. These confirmed study decision points, which were originally based on local testing, with 2 exceptions: 2 subjects had a Bethesda titer of 10.1 and 10.6 BU/mL at the time of randomization
ITI efficacy: overall success and time to success
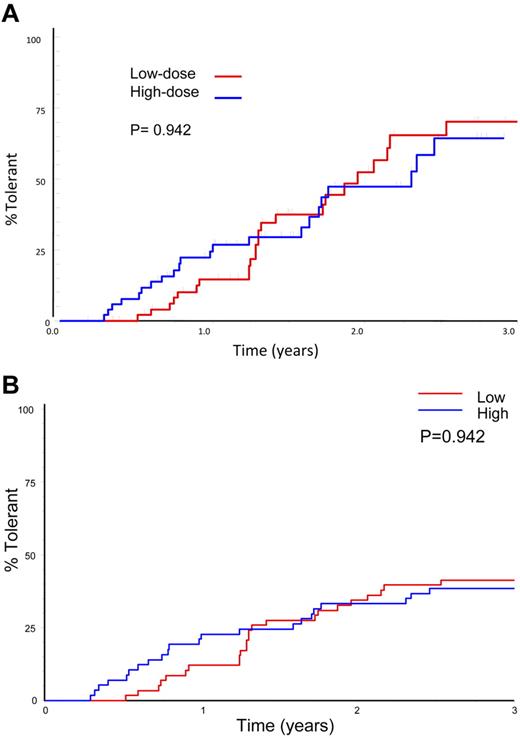
Sixty-six subjects reached an ITI success, partial success, or failure end point. Forty-six (69.7%) achieved tolerance, including 9 subjects who were still in the study at the prophylaxis phase. Three (4.5%) achieved partial success and 17 (25.8%, 12 HD) failed. A Kaplan-Meier plot of 66 subjects achieving a success, partial success, or failure end point showed no significant difference between treatment arms in the subjects achieving tolerance (70%) or in the time taken to achieve tolerance (Figure 3A; P = .096). A similar number of subjects achieved tolerance in each arm when all evaluable subjects were included in the analysis, including those lost to the study for logistic reasons: 24 of 58 (41%) LD and 22 of 57 (39%) HD (P = .909). An intention-to-treat Kaplan-Meier comparison of time to achieve tolerance, including all 115 randomized, treated subjects shows a lower success rate, with no significant difference in overall success rate or the time taken to achieve tolerance between treatment arms (Figure 3B; P = .942).
Time to success by treatment arm. (A) Kaplan-Meier plot showing the time to tolerance for the 66 patients who achieved a success, partial success, or failure end point, broken down by HD and LD treatment arm. (B) Intention-to treat Kaplan-Meier plot showing the time to tolerance for all 115 patients randomized and broken down by treatment arm. This plot shows no significant difference between treatment arms (P = .942), but a lower success rate because those not completing ITI or who were withdrawn for logistical reasons are also included.
Time to success by treatment arm. (A) Kaplan-Meier plot showing the time to tolerance for the 66 patients who achieved a success, partial success, or failure end point, broken down by HD and LD treatment arm. (B) Intention-to treat Kaplan-Meier plot showing the time to tolerance for all 115 patients randomized and broken down by treatment arm. This plot shows no significant difference between treatment arms (P = .942), but a lower success rate because those not completing ITI or who were withdrawn for logistical reasons are also included.
This sample size had the power to prove equivalence only with a 30% boundary of equivalence (1-tailed P = .05). Consequently, equivalent efficacy between HD and LD ITI could not be statistically established.
Phases 1 and 2 of ITI were significantly longer for the LD than for the HD subjects (Table 4; P = .027 and P = .002, respectively), although the overall time to achieve tolerance was not significantly different between the treatment arms (Table 4; P = .096).
Time to achieve ITI milestones by treatment arm reported as median (IQR) mo
| . | n . | LD . | n . | HD . | P . |
|---|---|---|---|---|---|
| Phase 1: start of ITI to negative titer | 29 | 9.2 (4.9-17.0) | 31 | 4.6 (2.8-13.8) | .017 |
| Phase 2: negative titer to first normal recovery | 27 | 13.6 (8.7-19.0) | 23 | 6.9 (3.5-12.0) | .001 |
| Phase 3: normal recovery to tolerance | 24 | 15.5 (10.8-22.0) | 22 | 10.6 (6.3-20.5) | .096 |
| . | n . | LD . | n . | HD . | P . |
|---|---|---|---|---|---|
| Phase 1: start of ITI to negative titer | 29 | 9.2 (4.9-17.0) | 31 | 4.6 (2.8-13.8) | .017 |
| Phase 2: negative titer to first normal recovery | 27 | 13.6 (8.7-19.0) | 23 | 6.9 (3.5-12.0) | .001 |
| Phase 3: normal recovery to tolerance | 24 | 15.5 (10.8-22.0) | 22 | 10.6 (6.3-20.5) | .096 |
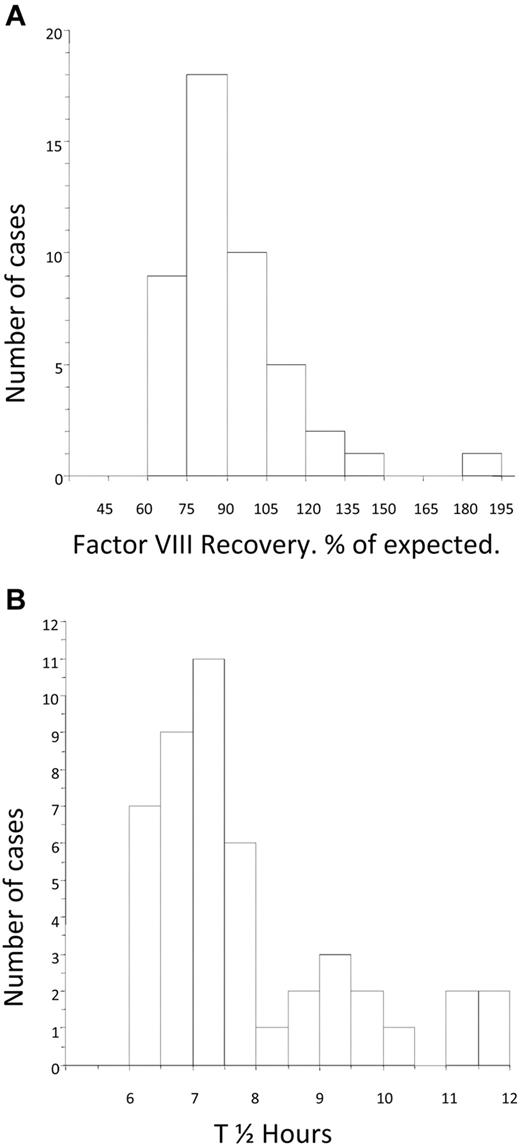
The mean FVIII recovery after tolerance was 91.6% (median, 86%; IQR, 77.3-99.7) of expected and was nearly normally distributed (Figure 4A). The mean post-tolerance half-life was 7.8 hours (median, 7.3 hours; IQR, 6.73-8.56). The distribution of half-life for the group was skewed toward the lower end of the normal range (Figure 4B). There was no difference in the post-tolerance half-life between the LD and HD arms (median, 7.39; IQR, 6.62-9.03 and median, 7.21; IQR, 6.84-7.77, respectively).
Distribution of factor VIII recovery and half-life at the time of tolerance. (A) Histogram showing the distribution of FVIII recovery in 46 patients who achieved tolerance, estimated at the point at which the subjects were considered tolerant. (B) Histogram showing the distribution of half-life in 46 patients who achieved tolerance, estimated at the point at which the subjects were first found to have a half-life in excess of 6 hours.
Distribution of factor VIII recovery and half-life at the time of tolerance. (A) Histogram showing the distribution of FVIII recovery in 46 patients who achieved tolerance, estimated at the point at which the subjects were considered tolerant. (B) Histogram showing the distribution of half-life in 46 patients who achieved tolerance, estimated at the point at which the subjects were first found to have a half-life in excess of 6 hours.
ITI efficacy: partial success and relapse
Three subjects (2 LD) had a partial response to ITI after 33 months of treatment. All responded clinically to FVIII without an increase in inhibitor titer. All had negative inhibitor titers but persistently diminished FVIII recoveries of 52.8%, 36.3%, and 49% of expected.
Six subjects partially relapsed, 2 transiently and 4 permanently, during the 12 months after tolerance induction (3 HD and 3 LD). Their median recovery and half-life at the time of tolerance was similar to the group as a whole (recovery median, 92.5%; IQR, 70.2-101.5 and half-life median, 7.0 hours; IQR, 6.0-9.0).
Relapse occurred at a median of 9.5 (IQR, 2-13) months from the time of tolerance. The inhibitor titer rose transiently to 1.4 BU/mL in 1 patient but remained undetectable in the remainder. FVIII recovery was reduced in 5 patients to 27%, 45%, 45%, 52.5%, and 61% of expected, and in 1 patient, recovery was normal (91%) but the half-life was decreased to 5.3 hours at the time of relapse. Two of 6 patients had normal pharmacokinetics by the end of the study and are now considered tolerant. The remaining 4 relapsed subjects continue to respond clinically to FVIII without anamnesis and therefore fulfill the criteria for partial response.
Variables influencing the outcome of ITI
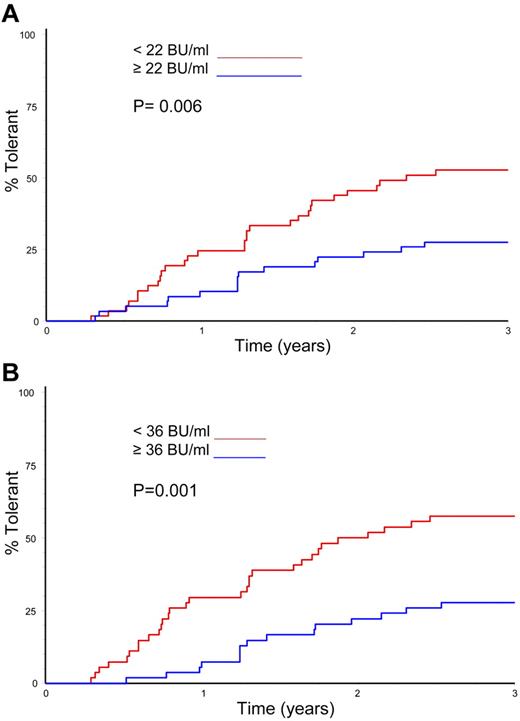
Logistic regression was used to analyze the effect of several variables on overall ITI success and time to successful tolerance (Table 5). The only variables significantly linked with successful ITI in the univariate analysis were the peak historical inhibitor titer and the peak titer on ITI (P = .026 and P = .002, respectively). In the multivariate analysis, however, only the peak inhibitor titer on ITI was a significant predictor of ITI outcome (P = .002). Time to successful ITI was also analyzed in a Kaplan-Meier comparison of peak historical titer, starting titer, and peak inhibitor titer on ITI; age at randomization; and presence or absence of infection. Again, only peak historical titer and peak titer on ITI significantly influenced the time taken to achieve tolerance (Figure 5A-B; P = .006 and P = .001).
Logistic regression: relationship between subject and treatment variables and the outcome of ITI
| Univariate analysis . | |
|---|---|
| Subject variable | P |
| Ethnicity (white/nonwhite) | .71 |
| Age at randomization (ITI) | .83 |
| Peak historical inhibitor titer | .026 |
| Peak titer on ITI | .002 |
| Peak titer on ITI ≤ 250 versus > 250 BU | .0002 |
| Time to titer of < 10 BU pre-ITI | .40 |
| Starting inhibitor titer | .98 |
| Treatment variable | |
| Randomized treatment arm | .82 |
| Protocol dose compliance | .35 |
| Product type | .58 |
| Total hospital in-patient days | .088 |
| CVAD in place | .58 |
| CVAD infection | .83 |
| Multivariate analysis | |
| Peak inhibitor titer on ITI | .002 |
| Univariate analysis . | |
|---|---|
| Subject variable | P |
| Ethnicity (white/nonwhite) | .71 |
| Age at randomization (ITI) | .83 |
| Peak historical inhibitor titer | .026 |
| Peak titer on ITI | .002 |
| Peak titer on ITI ≤ 250 versus > 250 BU | .0002 |
| Time to titer of < 10 BU pre-ITI | .40 |
| Starting inhibitor titer | .98 |
| Treatment variable | |
| Randomized treatment arm | .82 |
| Protocol dose compliance | .35 |
| Product type | .58 |
| Total hospital in-patient days | .088 |
| CVAD in place | .58 |
| CVAD infection | .83 |
| Multivariate analysis | |
| Peak inhibitor titer on ITI | .002 |
Time to tolerance by median peak inhibitor titres. (A) Kaplan-Meier plot showing the time to tolerance of all 115 randomized, treated subjects categorized according to peak historical titer above and below the median 22 BU/mL value (P = .006). (B) Kaplan-Meier plot showing the time to tolerance of all 115 randomized, treated subjects categorized according to peak inhibitor titer on ITI above and below the median 36 BU/mL value (P = .001).
Time to tolerance by median peak inhibitor titres. (A) Kaplan-Meier plot showing the time to tolerance of all 115 randomized, treated subjects categorized according to peak historical titer above and below the median 22 BU/mL value (P = .006). (B) Kaplan-Meier plot showing the time to tolerance of all 115 randomized, treated subjects categorized according to peak inhibitor titer on ITI above and below the median 36 BU/mL value (P = .001).
ITI morbidity: SAEs and nonserious adverse events
A total of 300 SAEs in 71 subjects were reported. Of these, 299 were categorized as SAEs only because they involved hospitalization. Thirty-eight (12.7%) were judged by the DSMB to be study related and 262 were deemed not to be study related. These SAEs and the 209 nonserious adverse events are summarized in Table 6.
SAEs and AEs
| Study-related SAEs, n =38 (12.7%) |
| Catheter-related events (n = 36) |
| 15 infections in 7 subjects |
| 13 insertions/removals |
| 5 malfunctioning catheters |
| 2 catheter site wound dehiscences |
| FVIII reaction (n = 1) |
| Trauma (n = 1) |
| Non-study–related SAEs, n = 262 (87.3%) |
| 11 traumas* |
| 69 spontaneous bleeds |
| 140 catheter-related problems |
| 75 catheter infections in 23 subjects |
| 43 catheter insertion/removals |
| 1 subclavian thrombosis |
| 3 hematomas at injection site |
| 21 other catheter-related problems |
| 10 infections unrelated to catheters |
| 12 fever of unknown origin |
| 9 surgeries (including 2 dental extractions) |
| 2 bronchospasm |
| 1 possible FVIII reaction |
| 5 unspecified |
| AEs (n = 209) |
| 64 catheter-related AEs |
| 80 minor infections |
| 29 fevers |
| 30 traumas |
| 6 unspecified |
| Study-related SAEs, n =38 (12.7%) |
| Catheter-related events (n = 36) |
| 15 infections in 7 subjects |
| 13 insertions/removals |
| 5 malfunctioning catheters |
| 2 catheter site wound dehiscences |
| FVIII reaction (n = 1) |
| Trauma (n = 1) |
| Non-study–related SAEs, n = 262 (87.3%) |
| 11 traumas* |
| 69 spontaneous bleeds |
| 140 catheter-related problems |
| 75 catheter infections in 23 subjects |
| 43 catheter insertion/removals |
| 1 subclavian thrombosis |
| 3 hematomas at injection site |
| 21 other catheter-related problems |
| 10 infections unrelated to catheters |
| 12 fever of unknown origin |
| 9 surgeries (including 2 dental extractions) |
| 2 bronchospasm |
| 1 possible FVIII reaction |
| 5 unspecified |
| AEs (n = 209) |
| 64 catheter-related AEs |
| 80 minor infections |
| 29 fevers |
| 30 traumas |
| 6 unspecified |
Includes 1 death from traumatic intracranial hemorrhage, prerandomization.
AE indicates nonserious adverse event.
ITI morbidity: CVAD infection
A total of 183 catheters were placed in 99 subjects, who had a median number of 1 catheter and a mean of 1.6 (range, 0-8) catheters. CVADs were placed for routine care before randomization in 73% of subjects. Eighty-two (45%) of the catheters were placed in 47 (81%) LD subjects, and the remainder were placed in 52 of 57 (91%) HD subjects. Eleven LD and 5 HD subjects were managed without a CVAD throughout the ITI. Of the 93 subjects for whom catheter type was identified, 72 (77%) were Port-a-Caths.
A total of 124 CVAD infections were reported among 41 subjects; 69 infections occurred among 19 subjects in the LD arm (median 2; mean 3.6 [range, 0-11 per subject]), and 22 HD subjects experienced a total of 55 infections (median 1, mean 2.5 [range 0-7 per subject]). In all, 58 (59%) subjects with catheters (28 LD and 30 HD) had no CVAD infection while on ITI.
The time from randomization to the first CVAD infection was similar in the 2 arms. CVAD infections were more common in patients with external CVADs (Broviac, Hickman, and peripheral IV lines) than with Port-a-Caths and similar implantable devices (implanted vs all non-implanted CVADs, P = .026).
The effect of central catheter infection on the outcome of ITI was analyzed in 93 of 99 subjects with a CVAD and adequate data. No difference was found in the number of infected subjects with catheters who achieved tolerance (44%; 9 of 19 LD and 9 of 22 HD) compared with those never infected (48%; 13 of 26 LD and 12 of 26 HD). Furthermore, there was no significant difference in the time taken by infected or noninfected subjects to reach phase 2 of ITI (7.6 vs 5.7 months), phase 3 (9.3 vs 9.4 months), or phase 4 of ITI (tolerance; 15.3 vs 14.9 months).
ITI morbidity: intercurrent bleeding
A total of 966 bleeding episodes were recorded for the entire cohort. Although the single death on the trial occurred from a traumatic intracranial hemorrhage before the start of ITI, only 65 (7%) of all recorded bleeds resulted in hospitalization and none was determined by the DSMB to be study related.
There were significantly more bleeds in the LD than in the HD treatment arm (Table 7), with a hazard ratio (HR) for all types of bleeds of 2.20 (P = .0019). The HR remained almost unchanged when 9 patients using bypass therapy prophylaxis were excluded from this analysis (HR 2.01, P = .016). Most (84.5%) bleeds occurred during phase 1 of ITI, with significantly more bleeding in the LD arm (P = .0046). Although more hemorrhaging was also observed in LD subjects throughout phases 2, 3, and 4 (Table 7), these differences were not significant. This pattern was seen for hemarthroses, muscle bleeds, and nonmusculoskeletal (other) bleeds when analyzed separately (data not shown).
All intercurrent bleeding by treatment arm and phase of ITI
| . | Regimen . | Bleeds, n . | HR . | 95% CI . | P . |
|---|---|---|---|---|---|
| All ITI (n = 58 vs 57) | LD | 684 | 2.2 | 1.34-3.62 | .0019 |
| HD | 282 | ||||
| Phase 1 (n = 58 vs 57) | LD | 573 | 2.27 | 1.29-4.01 | .0046 |
| HD | 241 | ||||
| Phase 2 (n = 27 vs 23) | LD | 47 | 3.4 | 0.84-13.8 | .088 |
| HD | 4 | ||||
| Phase 3 (n = 24 vs 22) | LD | 9 | 5.18 | 0.71-38.0 | .110 |
| HD | 3 | ||||
| Phase 4 (n = 24 vs 22) | LD | 54 | 1.70 | 0.80-3.63 | .170 |
| HD | 32 |
| . | Regimen . | Bleeds, n . | HR . | 95% CI . | P . |
|---|---|---|---|---|---|
| All ITI (n = 58 vs 57) | LD | 684 | 2.2 | 1.34-3.62 | .0019 |
| HD | 282 | ||||
| Phase 1 (n = 58 vs 57) | LD | 573 | 2.27 | 1.29-4.01 | .0046 |
| HD | 241 | ||||
| Phase 2 (n = 27 vs 23) | LD | 47 | 3.4 | 0.84-13.8 | .088 |
| HD | 4 | ||||
| Phase 3 (n = 24 vs 22) | LD | 9 | 5.18 | 0.71-38.0 | .110 |
| HD | 3 | ||||
| Phase 4 (n = 24 vs 22) | LD | 54 | 1.70 | 0.80-3.63 | .170 |
| HD | 32 |
Phase 1 indicates the time from the start of ITI until the Bethesda titer is negative; phase 2, from phase 1 until the FVIII recovery is normal; phase 3, from phase 2 to normal half-life; and phase 4, 12-month prophylactic phase after the half-life has normalized.
The difference in the number of bleeds between the treatment arms was largely attributable to a 2-fold difference in the bleeding rate per month (Table 8). The mean bleed rate in phase 1 was 0.623 bleeds/month in the LD arm and 0.282/month in the HD arm (P = .00024). The bleed rates in phases 2, 3, and 4 of ITI were much lower and were not significantly different between treatment arms (Table 8). Eight LD subjects and 21 HD subjects had no bleeding during the course of ITI (8 of 58 vs 21 of 57, P = .0085). There were 72 hospitalizations for bleeding in the LD arm and 39 in the HD arm (Mann-Whitney U test P = .145). The DSMB recommended immediate cessation of the study for reasons of safety on the basis of these data.
Intercurrent all bleed rate (bleeds/mo) by treatment arm and phase of ITI
| . | Dose (n) . | Mean . | Median . | IQR . | P . |
|---|---|---|---|---|---|
| Phase 1 | Low (58) | 0.623 | 0.564 | 0.093-0.886 | .00024 |
| High (57) | 0.282 | 0.000 | 0.000-0.440 | ||
| Phase 2 | Low (27) | 0.127 | 0.000 | 0.000-0.073 | .283 |
| High (22) | 0.087 | 0.000 | 0.000-0.000 | ||
| Phase 3 | Low (24) | 0.150 | 0.000 | 0.000-0.148 | .552 |
| High (22) | 0.033 | 0.000 | 0.000-0.000 | ||
| Phase 4 | Low (24) | 0.175 | 0.150 | 0.000-0.290 | .112 |
| High (22) | 0.102 | 0.000 | 0.000-0.231 |
| . | Dose (n) . | Mean . | Median . | IQR . | P . |
|---|---|---|---|---|---|
| Phase 1 | Low (58) | 0.623 | 0.564 | 0.093-0.886 | .00024 |
| High (57) | 0.282 | 0.000 | 0.000-0.440 | ||
| Phase 2 | Low (27) | 0.127 | 0.000 | 0.000-0.073 | .283 |
| High (22) | 0.087 | 0.000 | 0.000-0.000 | ||
| Phase 3 | Low (24) | 0.150 | 0.000 | 0.000-0.148 | .552 |
| High (22) | 0.033 | 0.000 | 0.000-0.000 | ||
| Phase 4 | Low (24) | 0.175 | 0.150 | 0.000-0.290 | .112 |
| High (22) | 0.102 | 0.000 | 0.000-0.231 |
Bypass-therapy prophylaxis was used at the managing physician's discretion. Seven LD subjects and 2 HD subjects were treated with bypass-therapy prophylaxis in standard dosage for between 3 weeks and 19 months during phase 1 of ITI. We currently have insufficient data to evaluate the relative efficacy of prophylaxis in this group. The comparative pharmaco-economic analysis of the 2 treatment arms is ongoing and will be reported separately.
Discussion
We report the results of the first prospective, randomized trial of ITI in patients with severe hemophilia A and high titer inhibitors. We hypothesized that HD ITI would achieve tolerance more rapidly than LD ITI but would have a similar overall success rate. As expected, the success rate was statistically similar between treatment arms. However, the study lacked the statistical power required by a comparative trial to demonstrate therapeutic equivalence below the 30% boundary of equivalence.
The tolerance rate reported for this study is lower than that reported in many other studies.1-9 However, this is the first ITT analysis of immune tolerance and includes subjects unable or unwilling to complete ITI, reflecting the previously unreported treatment adherence difficulties encountered in normal clinical practice. Furthermore, because of the fixed and well-defined study end points required of a comparative trial, subjects who did not achieve the determinants of success after 33 months on ITI or whose titers declined more slowly than what was allowable for the study were reported as failures, although some may have subsequently achieved tolerance through continued treatment off-study. Furthermore, the 12% of subjects determined in this trial to be partial responders would have been classified as “successes” by the less stringent end points used in previous studies. For example, although the NAITR reported an 83% success rate among good-risk subjects, 87% of registry participants were so defined solely on the basis of a negative inhibitor titer.9 For these reasons, the success rate reported in this trial is not directly comparable with earlier uncontrolled, retrospective studies.
The time to tolerance for the entire study cohort was similar to that reported previously. However, HD subjects achieved a negative titer and normal recovery significantly more rapidly than LD subjects, and LD subjects required 50% longer to achieve tolerance overall (nonsignificant). The clinical implications of these findings are unclear at this time. Although prolonged ITI could conceivably adversely affect adherence to therapy, treatment compliance and drop-out rate did not differ between the treatment arms.
Tolerance was defined as the restoration of normal FVIII pharmacokinetics using a consensus definition. A subsequent report showed that the median half-life and recovery in 52 subjects with a median age of 3.1 ± 1.5 years was 9.88 ± 1.89 hours and 1.90 ± 0.43 IU/dL, respectively. These data were normally distributed, but reflected considerable interpersonal variation. Between the ages of 1 and 6 years, half-life increased by 0.4 hours per year.37 At the time of tolerance, our subjects (mean age, 15.4 months) had a mean FVIII recovery of 90.9% of expected (IQR, 66-182.9), normally distributed, and a mean half-life of 8.0 (IQR, 6.73-8.56; Figure 4A-B). Although comparable with patients of similar age lacking inhibitors,37 the half-life estimations of “tolerant” subjects in our trial were skewed toward the protocol-prescribed lower limit, either reflecting the comparatively younger age of our cohort or suggesting that some subjects may have persistently very low-level inhibitor activity that did not compromise response to FVIII replacement.
We also report the first prospectively collected data on the loss of previously normalized pharmacokinetics during the first year after successful tolerance, defined in this study as relapse. Four of 37 (11%) tolerized subjects exhibited a nonrecovered loss of normalized pharmacokinetics at variable times during the prophylaxis phase of study, becoming partial responders. Pharmacokinetics in this group were indistinguishable from the group as a whole when they were originally considered tolerant. Although the NAITR reported a 12% relapse rate as part of its retrospective analysis, those patients had completely lost FVIII responsiveness and thus are not comparable.38
One of the objectives of the I-ITI study was to investigate potential host- and treatment-related predictors of ITI success in good-risk subjects using a logistical regression analysis of the effect of these variables on outcome (Table 7). This confirmed historical peak inhibitor titer and peak titer on ITI to be significantly inversely associated with ITI outcome in a univariate analysis. In a multivariate analysis, however, only peak titer on ITI remained a significant determinant of ITI success. These data are consistent with previous reports that a lower historical peak titer,7,9 particularly a lower peak titer on ITI, are significant predictors of successful ITI outcome.9
The study design and subject demographics prevented us from confirming the previously reported relationship between low starting inhibitor titer and successful ITI outcome, because our subjects all had a starting titer < 10 BU/mL at the time of randomization.(Table 7). A pre-ITI titer of < 10 BU primarily defined the good-risk ITI subject in both the IITR and NAITR, and was therefore an important inclusion criterion for this trial.7,9 This remained a controversial enrollment requirement throughout the trial because of concern among some investigators about the potential of inhibitor-related morbidity during the waiting period for inhibitor titer decline. We found that it took a median of only 5 months for the inhibitor to decline to < 10 BU/mL and < 9 months for most subjects with historical titers < 200 BU/mL. However, a similar outcome was observed in subjects with a starting titer above and below the median of 6 BU, suggesting that deferring ITI until very low starting inhibitor titers are achieved results in no further improvement in ITI outcome. This contrasts with NAITR data suggesting that extremely low starting titers were associated with a uniformly good outcome.9
ITI initiation at an early age was an important determinant of ITI success in the IITR,7 but not in the much younger cohort reported in the NAITR.9 Most subjects in the present study were < 2 years old, with a restricted age range. No significant advantage of initiation of ITI at an early age could be demonstrated for this reason.
An important aim of the present trial was to establish the morbidity of ITI and to compare the morbidity associated with HD and LD ITI, because there are very few published data on this subject. More than 50% of SAEs reported herein were CVAD-related events, and only 13% were determined by the DSMB to be study related. The high prevalence of CVAD-related adverse events during ITI has already been reported in retrospective analyses,15,17 and an adverse effect of CVAD infection on the outcome of ITI has also been suggested.
In the I-ITI study, although 86% of all subjects received ITI through at least one CVAD, 73% had a catheter placed for routine treatment of bleeding before the initiation of ITI. Catheter use did not differ significantly between treatment arms. Unexpectedly, we also observed no differences in the rates of CVAD infection between treatment arms. Although ITI was administered by peripheral venipuncture in twice as many LD subjects (n = 11) as HD subjects (n = 5), this difference was not significant. Moreover, 45% of all catheters were placed in subjects receiving LD ITI. Implantable devices were significantly less likely to become infected than external catheters, as reported previously.15
Contrary to expectations, we observed no significant impact of CVAD infection on either overall ITI success or the time taken to achieve ITI milestones. CVAD infection has long been anecdotally associated with a nonspecific but sometimes dramatic increase in inhibitor titer and sometimes subsequent failure of previously promising ITI induction. Although not observed in this good-risk cohort, the potential adverse effect of catheter infection on ITI outcome will be further evaluated in poor-risk ITI subjects as part of the ongoing RESIST study.39
Although ITI induction has anecdotally been observed to offer some protection from intercurrent bleeding, this has never been systematically investigated in a large cohort. The prospective nature of this trial allowed us to collect reliable data to explore the effect of FVIII dose on bleeding frequency. In all, 966 hemorrhagic episodes were reported during this trial. A single non-study–related hemorrhagic death occurred before the start of ITI. Only 7% of all recorded bleeds resulted in hospitalization, however, none was considered by the DSMB to be study related.
We were surprised to discover a significantly greater number of bleeds with LD compared with HD ITI. Furthermore, significantly more LD subjects required hospitalization for bleeding and significantly fewer experienced a bleed-free course on ITI compared with their HD counterparts. The increased number of bleeds was caused by an increase in bleeding rate and not by the greater duration of phase 1 LD ITI or the disproportionate use of bypass therapy prophylaxis during HD ITI. The difference in bleed rate between arms was most marked during phase 1 of ITI, when 85% of bleeding occurred and when the FVIII half-life was presumably the shortest. During phase 2 and 3, the bleeding rate declined dramatically in both arms. This pattern was seen for hemarthrosis, muscle bleeds, and soft tissue bleeding. These data imply that both HD and LD regimens provide some degree of protection from intercurrent bleeding when the inhibitor has fallen to a low level. More bleeding was observed in LD than in HD subjects throughout the study, and this persisted through prophylaxis.
Detailed pharmaco-economic analysis and modeling of the results of the I-ITI study may influence the choice of optimal regimen. However, one of the major goals of inhibitor eradication is to minimize lifelong bleeding-related morbidity. Consequently, although global access to ITI is assumed to require the availability of less aggressive FVIII infusion strategies, the future clinical practice of ITI must prioritize early and effective control of bleeding. The prospective study of strategies to minimize bleeding during ITI (eg, the ENJOIH study) may be a critical next step in optimizing ITI.40
The DSMB recommended study termination because they identified bleeding as a safety issue. They also recommended no further recruitment because the power calculation showed that proof of therapeutic equivalence between the 2 FVIII doses being compared using a 20%, 15%, or 10% boundary of equivalence would have required 75, 132, or 297 subjects completing each treatment arm. This was not feasible despite recruitment from 90 centers in 17 countries.
Barriers associated with the conduct of clinical trials in rare disease populations, particularly those relevant to study logistics and subject recruitment, represented major challenges during this trial. Alternative models for clinical trial design may indeed be useful for the design of future interventional trials. Nonetheless, this study has created a precedent and a model that has encouraged other investigators to initiate multinational, investigator-led trials in hemophilia, a trend that we hope will continue.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Dr Evelien Mauser-Bunschoten for her contribution to study design and support throughout the trial; Ilene Goldberg, Rob Hollingsworth, Janet Goldstone, and Matt Foulkes for research coordination and day-to-day administration of the study; Dr Ric Swindell of the Christie Hospital Clinical Trials Unit (Manchester, United Kingdom) for the statistical analysis of the data and review the final manuscript; Ernie Gascoigne from BPL Limited for the pharmacokinetic analyses; Dr Keith Hoots and Marisella Trujillo (Gulf States Pharmacy, Houston, TX) for the independent management of the US factor subsidy; and Drs Wander Van Heerde and Bert Verbruggen of Rathboud University (Nijmegen, The Netherlans) for the timely performance of central inhibitor testing.
The authors further acknowledge the following:
The I-ITI Steering Committee for their valuable input into study development, management, and analysis, which included at one time or another: Tom Abshire, Gunter Auerswald, Steve Deitcher, Graham Dunn, Nadia Ewing, Anders Glomstein, Alessandro Gringeri, Paul Harper, Nino Haya, Keith Hoots, Jorgen Ingerslev, Gili Kenet, Tom Kisker, Christoph Male, P. M. Mannucci, Uri Martinowitz, Evelein Mauser-Bunschotten, Bill McWhirter, Claude Negrier, Paul Ockleford, Kathelijne Peerlinck, Pia Petrini, George-Etienne Rivard, Elena Santagostino, Ben Saxon, Marti Siimes, Alison Street, Joan Tusell, Lochie Teague, Mareike van den Berg, Christine Van Geet, and Akira Yoshiojka.
The Data Safety Monitoring Committee for all of their diligent safety oversight of this trial: Professors L. M. Aledort (chair), Alan Giles, and Inge Scharrer.
Science and Publications Committees: Tom Kisker (co-chair), Georges Rivard (co-chair), Leon Hoyer, David Lillicrap, Mareike van den Berg, and Akira Yoshioka (2002-2011); Keith Hoots (chair), Victor Blanchette, Frank Hill, Claude Negrier, Ulrike Nowak-Gottl, and Allison Street (2011-present).
The participating investigators for their strong support of this trial from its inception: Thomas C. Abshire, Geoff Allen, Irmel Ayala, José Antonio Aznar, Dorothy Barnard, Carolyn Bennett, Manuel Carcao, Alice J. Cohen, Peter Collins, Dr Christine Demers, Amy Dunn, Miguel Escobar, Nadia Ewing, Brian Feldman, Frances Flug, Joan Gill, Anders Glomstein, Jenny Goudemand, Gunwant Guron, John Hanley, Margaret Heisel, Keith Hoots, Jeffrey Hord, Anne Hurlet, Eisaburo Ishii, David Keeling, Gili Kenet, Bryce Kerlin, Dr Robert Klaassen, Maseo Kobayashi, Ryokji Kobayashi, Roshi Kulkarni, Cindy A. Leissinger, Lianne Lockwood, Marilyn Manco-Johnson, Catherine Manno, Prasad Mathew, Tadashi Matsushita, Evelien Mauser-Bunschotten, Paul E Monahan, Massimo Morfini, Anne Neff, Ellis Neufeld, Diane Nugent, Chiai Oi, Yuri Okimoto, Rafael Parra, Kathelijne Peerlinck, Dr Steven Pipe, Man-Chiu Poon, Leslie Raffini, Margaret Ragni, Michael Recht,Ulrike Reiss, Bruce Ritchie, Dr Georges-Étienne Rivard, Vilamarie Rodriguez, Cathy Rosenfield, Anne Rossi, Elena Santagostino, Frank Shafer, Amy Shapiro, Midori Shima, Akira Shimada, Prof Akira Shirahata, Takashi Suzuki, Kyoko Takeichi, Michael Tarantino, A. E. Thomas, Alexis Thompson, Ikuya Usami, Tamaki Ueno, Leonard A. Valentino, Mareike van den Berg, Raymond Watts, Eric J. Werner, Brian Wicklund, and all study coordinators.
The study sponsors who supported this investigator-led study through unrestricted grants for study administration and through FVIII product subsidies in Japan, the United States, and Argentina and for their support and encouragement for the project from its inception: Bayer, Baxter, C.S.L. Behring, Grifols, Pfizer, and the Japanese Red Cross.
Authorship
Contribution: C.R.M.H. and D.M.D. were co–principal investigators of this study and were jointly responsible for study design and oversight, analysis of data, and preparation of the manuscript.
Conflict-of-interest disclosure: C.R.M.H. has received unrestricted research/educational funding from Bayer, Baxter, Pfizer, and CSL-Behring. D.M.D. declares no competing financial interests.
The current affiliation for D.M.D. is Division of Blood Diseases and Resources at the National Heart, Lung, and Blood Institute, Bethesda, MD.
Correspondence: C. R. M. Hay, Manchester University Dept of Haematology, Manchester Royal Infirmary, Oxford Road, Manchester, United Kingdom M13 9WL; e-mail: haemophilia@man.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal