Abstract
Neutrophil extracellular traps (NETs) are DNA structures released by dying neutrophils and claimed to constitute a new microbicidal mechanism. Killing by NET-forming cells is ascribed to these structures because it is prevented by preincubation with DNase, which has been shown to dismantle NETs, before addition of the target microorganisms. Curiously, the possibility that the microorganisms ensnared in NETs are alive has not been considered. Using Staphylococcus aureus and Candida albicans blastospores, we demonstrate that the microorganisms captured by NETs and thought to be killed are alive because they are released and recovered in cell medium by incubation with DNase. It is concluded that NETs entrap but do not kill microbes.
Introduction
Among the strategies that neutrophils use to kill invading microorganisms, great emphasis has recently been placed on the role of the so-called neutrophil extracellular traps (NETs), which are considered part of the neutrophil response to microbes. Activated neutrophils die and release these structures in the extracellular space. They are composed of decondensed chromatin decorated with antimicrobial proteins that, reportedly, trap and kill a broad range of microbes.1,2 Phorbol 12-myristate 13-acetate (PMA) is considered the most potent agent to induce NET formation.3 The basic methodology used to measure killing by NETs entails: (1) preincubation of neutrophils with PMA on plastic plates; (2) incubation without PMA, in fresh medium containing 2% heat-inactivated pooled human serum without or with DNase I, which has been shown to dismantle NETs1 ; (3) addition of microorganisms and centrifugation; (4) incubation for 20 or 30 minutes; and (5) collection of aliquots of cell medium and plating to determine colony-forming units (CFU).
The decreased number of microorganisms found in the medium of cells preincubated with PMA is considered as evidence of extracellular killing because it is not inhibited by cytochalasin D (CD) that blocks phagocytosis. This killing is attributed to the NETs because it is prevented by incubation with DNase before addition of microorganisms. Parallel samples are often carried out with cells that are not preincubated with PMA to determine intracellular killing. Killing that is inhibited by CD and unaffected by DNase is defined as phagocytic.
The basic assumption of the assay, as it is conceived, is that the microorganisms missing from the culture medium of cells that formed NETs during preincubation with PMA have been captured and killed by these structures. However, there is a big question mark about the reliability of this conclusion because, to our knowledge, there is no formal proof that microbes trapped in NETs are, indeed, dead. This prompted us to set up experiments to verify the correctness of this assumption.
Methods
Reagents
Percoll, DNase I type IV, CD, gelatin from calf skin, type IV, and PMA were purchased from Sigma-Aldrich.
Isolation of neutrophils
Neutrophils were isolated from peripheral blood drawn from human volunteers after obtaining their written informed consent, by centrifugation over Percoll4 and resuspended in RPMI medium supplemented with 10mM HEPES and 2% heat-inactivated human serum.
Phagocytic and NET killing
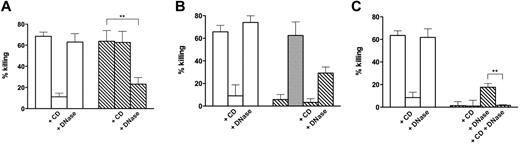
For bactericidal activity, the method described by Fuchs et al2 was adopted, with slight modifications. Staphylococcus aureus was grown overnight in Luria-Bertani broth. On the day of the experiment, an aliquot of microorganism culture was diluted in Luria-Bertani broth, grown for 2 to 3 hours, washed, and suspended in RPMI-HEPES. Sterile 24-well plates (Greiner Bio-One) were used. Human neutrophils (106/well in 0.5 mL) were stimulated for 120 minutes at 37°C with PMA (20 ng/mL) to form NETs, or left unstimulated. In our experimental conditions, formation of NETs containing extracellular strands of DNA with granule proteins attached was detected after staining with Hoechst 33258 and immunofluorescence (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Subsequently, the medium was carefully removed, and the cells were further incubated for 20 minutes in fresh medium without PMA, with or without 100 U/mL DNase I or CD (10 μg/mL). The cultures were infected with 106S aureus (bacteria to cell ratio: 1) centrifuged for 10 minutes at 800g, and further incubated for 20 minutes. After agitation at medium/high speed for 2 minutes in a microplate mixer, aliquots were taken, sequentially diluted in H2O, adjusted to pH 11 and 0.9% NaCl, and plated to determine CFU as described.5 The results of these experiments are shown in Figure 1A. At the end of the 20-minute incubation with bacteria, DNase I (100 U/mL) was added to the wells not already containing it, and incubation continued for an additional 10 minutes. CFUs were determined as per the procedure described in the preceding paragraph. The results of these experiments are shown in Figure 1B. Finally, after the 10-minute incubation with DNase, the cell medium was aspirated, and the wells were washed with H2O adjusted to pH 11 to determine the CFU of bacteria remaining in the wells. The number of bacteria recovered from the washings was added to the number of bacteria obtained after DNase treatment, shown in Figure 1B, and used to construct Figure 1C.
Killing of S aureus by control and NET-forming neutrophils. (A) S aureus was added to control neutrophils (white bars) and neutrophils forming NETs after incubation for 120 minutes with 20 ng/mL PMA (hatched bars). CD (10 μg/mL), DNase I (100 U/mL), or both (C) were added 20 minutes before addition of bacteria. (B) Same as panel A, with killing calculated after DNase I (100 U/mL) was added at the end of incubation with bacteria to the wells not already containing it. Gray bar represents killing calculated after addition of heat-inactivated (10 minutes, 70°C) DNase. (C) Same as panel B, with killing calculated using the total number of bacteria in the wells (ie, bacteria in the cell medium plus bacteria recovered from the wells after aspiration of the cell medium and washing). Error bars represent SEM (n = 4-6). With heat-inactivated DNase (n = 3). **P < .01 (Student t test on paired data).
Killing of S aureus by control and NET-forming neutrophils. (A) S aureus was added to control neutrophils (white bars) and neutrophils forming NETs after incubation for 120 minutes with 20 ng/mL PMA (hatched bars). CD (10 μg/mL), DNase I (100 U/mL), or both (C) were added 20 minutes before addition of bacteria. (B) Same as panel A, with killing calculated after DNase I (100 U/mL) was added at the end of incubation with bacteria to the wells not already containing it. Gray bar represents killing calculated after addition of heat-inactivated (10 minutes, 70°C) DNase. (C) Same as panel B, with killing calculated using the total number of bacteria in the wells (ie, bacteria in the cell medium plus bacteria recovered from the wells after aspiration of the cell medium and washing). Error bars represent SEM (n = 4-6). With heat-inactivated DNase (n = 3). **P < .01 (Student t test on paired data).
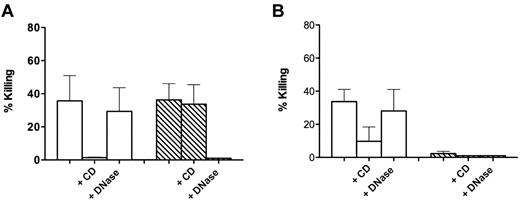
For fungicidal activity, Candida albicans blastospores were grown as described5 and suspended in 0.9% NaCl solution. The experimental procedure was similar to that used with S aureus with the following modifications: (1) the wells were precoated with 1% bovine gelatin as this treatment was shown to prevent Candida adhesion6 ; (2) the cultures were infected with 5 × 104C albicans (yeasts to cell ratio: 0.05), centrifuged for 10 minutes at 200g, and further incubated for 30 minutes; (3) at the end of incubation, aliquots of the cell medium were taken after gentle pipetting; and (4) after the 10-minute treatment, with DNase subsequent to the 30-minute incubation with the yeasts, aliquots of the incubation medium were taken after vigorous pipetting.
Results and discussion
Figure 1A shows the results of killing experiments carried out as reported in the literature, with control neutrophils and neutrophils that formed NETs after preincubation with PMA.2 The results are comparable with those already published in that: (1) killing by control cells was inhibited by CD, indicating phagocytosis-dependent killing, and, as expected, was not affected by DNase pretreatment; and (2) killing by cells induced to form NETs by preincubation with PMA was not affected by CD and was inhibited by DNase treatment before addition of bacteria, indicating its extracellular nature and dependence on NETs.
To verify whether the bacteria captured by NETs were actually dead, at the end of the 20-minute incubation with neutrophils, DNase I was added to the wells and incubation was continued for an additional 10 minutes before the collection of aliquots to determine CFU. Figure 1B shows that, after this procedure, in cells that formed NETs, killing was considerably reduced, indicating that live bacteria were released in cell incubation media after DNase treatment. The recovery of viable microorganisms did not occur using heat-inactivated DNase added after incubation with bacteria (Figure 1B gray bar), indicating that enzymatically active DNase is required for their release.
Killing was completely absent when the microorganisms remaining in the wells after removal of the medium were recovered by washing and considered in the calculations (Figure 1C). Results similar to those reported in Figure 1 were obtained even after a 5-fold increase in the bacteria to cell ratio (supplemental Figure 2) and by prolonging the incubation time to 90 minutes (supplemental Figure 3). Note that the results of supplemental Figures 2 and 3 are expressed as percentage survival because in these experiments bacterial growth was observed. Supplemental Figure 3A shows that the “trapping” effect is more pronounced after 90 minutes of incubation (compare with Figure 1A) and results in a reduced survival. However, after DNase treatment and washing of the wells, killing was no more apparent and, indeed, bacterial growth was observed (supplemental Figure 3B).
The residual killing (∼ 20%) by NET-forming cells that were treated with DNase before incubation with bacteria (Figure 1A,C bar 6; Figure 1B bar 7) probably represents phagocytic killing because it was inhibited by CD (Figure 1 C last bar). The fact that a phagocytic killing was observed only when NETs were dismantled by preincubation with DNase suggests that entrapment of bacteria by these structures might hinder phagocytosis.
Almost identical results were obtained in experiments in which C albicans was used as a target (Figure 2). We could confirm a killing dependent on NETs that was prevented by preincubation with DNase (Figure 2A). However, similarly to what observed for S aureus, such a killing proved to be apparent, and failure to recover viable microorganisms was a consequence of trapping in DNA-based structures because addition of DNase at the end of incubation of the Candida blastospores with NET-forming cells caused their release and complete recovery in the incubation medium (Figure 2B).
Killing of C albicans by control and NET-forming neutrophils. (A) C albicans was added to control neutrophils (white bars) and neutrophils forming NETs after incubation for 120 minutes with 20 ng/mL PMA (hatched bars). CD (10 μg/mL) and DNase I (100 U/mL) were added 20 minutes before addition of bacteria. (B) Same as panel A, with killing calculated after DNase I (100 U/mL) was added at the end of incubation with the yeasts to the wells not already containing it. Error bars represent SEM (n = 3).
Killing of C albicans by control and NET-forming neutrophils. (A) C albicans was added to control neutrophils (white bars) and neutrophils forming NETs after incubation for 120 minutes with 20 ng/mL PMA (hatched bars). CD (10 μg/mL) and DNase I (100 U/mL) were added 20 minutes before addition of bacteria. (B) Same as panel A, with killing calculated after DNase I (100 U/mL) was added at the end of incubation with the yeasts to the wells not already containing it. Error bars represent SEM (n = 3).
Our data demonstrate that no microbicidal activity is observed in association with NETs and that microorganisms entrapped in these structures are alive. We suggest a more ordinary function for NETs (ie, ensnaring bacteria to prevent dissemination of microorganisms and wall off the infection). A similar function, inhibition of phagocytosis and bacterial containment, has been attributed to fibrin.7 Additional studies are required to establish whether, and under what conditions, NETs may play a role in microbial killing.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Maurizio Romano for skillful assistance in fluorescence microscopy.
This work was supported by the Italian Telethon Foundation (contract grant GGP07221; P.D.).
Authorship
Contribution: R.M. and E.D. designed and performed experiments and wrote the manuscript; and P.D. supervised and designed the experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pietro Dri, Department of Life Sciences, Via Valerio, 28, Ed R, 34127 Trieste, Italy; e-mail: dri@units.it.
References
Author notes
R.M and E.D. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal